Influence of Deposition Parameters of TiO2 + CuO Coating on the Membranes Surface Used in the Filtration Process of Dairy Wastewater on Their Functional Properties
Abstract
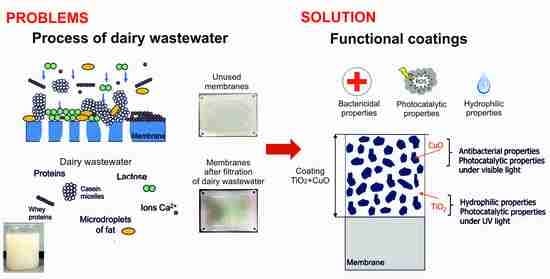
:1. Introduction
2. Materials and Methods
2.1. MS-PVD Process
2.2. Surface Structure and Chemical Composition of the Coatings
2.3. Antibacterial Test
2.4. Photocatalytic Properties
2.5. Surface Free Energy
2.6. Filtration and Separation Performance
3. Results and Discussion
3.1. Structure and Elemental Composition Characterization
3.2. Antibacterial Test
3.3. Photocatalytic Properties
3.4. Surface Free Energy
3.5. Filtration and Separation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kołtuniewicz, A.B.; Drioli, E. Membranes in Clean Technologies. In Theory and Practice; WILEY-VCH Verlag GmbH & Co: Weinheim, Germany, 2008; Volume 1–2. [Google Scholar]
- Hausmann, A.; Sanciolo, P.; Vasiljevic, T.; Weeks, M.; Schroën, K.; Gray, S.; Duke, M. Fouling of dairy components on hydrophobic polytetrafluoroethylene (PTFE) membranes for membrane distillation. J. Membr. Sci. 2013, 442, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of Nanocomposite Membranes for Water Treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Maddah, H.; Chogle, A. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2017, 7, 2637–2651. [Google Scholar] [CrossRef] [Green Version]
- Vadi, P.K.; Rizvi, S.S.H. Experimental evaluation of a uniform transmembrane pressure crossflow microfiltration unit for the concentration of micellar casein from skim milk. J. Membr. Sci. 2001, 189, 69–82. [Google Scholar] [CrossRef]
- Walstra, P.; Geurts, T.J.; Noomen, A.; Jellema, A.; van Boeke, M.A.J.S. Dairy Technology: Principles of Milk Properties and Processes; Marcel Dekker: New York, USA, 1999. [Google Scholar]
- Berg, T.H.A.; Knudsen, J.C.; Ipsen, R.; Berg, F.; Holst, H.; Tolkach, A. Investigation of Consecutive Fouling and Cleaning Cycles of Ultrafiltration Membranes Used for Whey Processing. Int. J. Food Eng. 2014, 10, 367–381. [Google Scholar] [CrossRef]
- Sabri, S.; Najjar, A.; Manawi, Y.; Eltai, N.O.; Al-Thani, A.; Atieh, M.A.; Kochkodan, V. Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum. Membranes 2019, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Subramani, A.; Hoek, E. Direct observation of initial microbial deposition onto reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2008, 319, 111–125. [Google Scholar] [CrossRef]
- Ozdemir, S.S.; Buonomenna, M.G.; Drioli, E. Catalytic polymer membranes: Preparation and application. Appl. Catal. 2006, 307, 167–183. [Google Scholar] [CrossRef]
- Liu, L.; Di, D.W.; Park, H.; Son, M.; Hur, H.-G.; Choi, H. Improved antifouling performance of polyethersulfone (PES) membrane via surface modification. RSC Adv. 2015, 5, 7340–7348. [Google Scholar] [CrossRef]
- Misdan, N.; Ismail, A.F.; Hilal, N. Recent advances in the development of (bio)fouling resistant thin film composite membranes for desalination. Desalination 2016, 380, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Wang, Z.; Wu, J.; Wang, Y.; Wang, J.; Wang, S. A green strategy to immobilize silver nanoparticles onto reverse osmosis membrane for enhanced anti-biofouling property. Desalination 2017, 401, 32–41. [Google Scholar] [CrossRef]
- Aryanti, P.T.P.; Sianipar, M.; Zunita, M.; Wenten, I.G. Modified membrane with antibacterial properties. Membr. Water Treat. 2017, 8, 463–481. [Google Scholar]
- Kowalik-Klimczak, A.; Stanisławek, E.; Kacprzyńska-Gołacka, J.; Osuch-Słomka, E.; Bednarska, A.; Skowroński, J. The polyamide membranes functionalized by nanoparticles for biofouling control. Desalin. Water Treat. 2018, 128, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Bojarska, M.; Nowak, B.; Skowroński, J.; Piątkiewicz, W.; Gradoń, L. Growth of ZnO nanowires on polypropylene membrane surface—Characterization and reactivity. Appl. Surf. Sci. 2017, 391, 457–467. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Stanisławek, E.; Kacprzyńska-Gołacka, J. The surface modification of polyamide membranes using graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124281. [Google Scholar] [CrossRef]
- Ren, P.-F.; Fang, Y.; Wan, L.-S.; Ye, X.-Y.; Xu, Z.-K. Surface modification of polypropylene microfiltration membrane by grafting poly(sulfobetaine methacrylate) and poly(ethylene glycol): Oxidative stability and antifouling capability. J. Membr. Sci. 2015, 492, 249–256. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.L.; Sugumaran, J.; Shoparwe, N.F. Antifouling Properties of PES Membranes by Blending with ZnO Nanoparticles and NMP–Acetone Mixture as Solvent. Membranes 2018, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xu, Y.; Chen, S.; Li, J.; Han, W.; Sun, X.; Wu, D.; Hu, Z.; Wang, L. Enhanced antifouling and antibacterial properties of poly (ether sulfone) membrane modified through blending with sulfonated poly (aryl ether sulfone) and copper nanoparticles. Appl. Surf. Sci. 2018, 434, 806–815. [Google Scholar] [CrossRef]
- Bi, Y.; Han, B.; Zimmerman, S.; Perreault, F.; Sinha, S.; Westerhoff, P. Four release tests exhibit variable silver stability from nanoparticle-modified reverse osmosis membranes. Water Res. 2018, 143, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. Thermodynamic aspects of contact angle hysteresis. Adv. Colloid Interface Sci. 1994, 50, 121–141. [Google Scholar] [CrossRef]
- Chetana, P.; Srinatha, B.; Somashekar, M.; Policegoudra, R. Synthesis, spectroscopic characterisation, thermal analysis, DNA interaction and antibacterial activity of copper(I) complexes with N,N′-disubstituted thiourea. J. Mol. Struct. 2016, 1106, 352–365. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Phan, D.-N.; Dorjjugder, N.; Saito, Y.; Khan, M.Q.; Ullah, A.; Bie, X.; Taguchi, G.; Kim, I.-S. Antibacterial mechanisms of various copper species incorporated in polymeric nanofibers against bacteria. Mater. Today Commun. 2020, 25, 101377. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.; Navío, J. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Chi, M.; Hu, W.; Zhang, N.; He, W. Porous Polymer-Titanium Dioxide/Copper Composite with Improved Photocatalytic Activity toward Degradation of Organic Pollutants in Wastewater: Fabrication and Characterization as Well as Photocatalytic Activity Evaluation. Catalysts 2020, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Navabpour, P.; Cooke, K.; Sun, H. Photocatalytic Properties of Doped TiO2 Coatings Deposited Using Reactive Magnetron Sputtering. Coatings 2017, 7, 10. [Google Scholar] [CrossRef] [Green Version]














| Power of Ti Magnetron Source [W] | Power of Cu Magnetron Source [W] | Atmosphere | Pressure [mbar] | Time of the Process [s] |
|---|---|---|---|---|
| 650 | 15 | 10% O2 + 90% Ar | 5.0 × 10−3 | 30 |
| 650 | 100 | 10% O2 + 90% Ar | 5.0 × 10−3 | 30 |
| 650 | 200 | 10% O2 + 90% Ar | 5.0 × 10−3 | 30 |
| 1000 | 15 | 10% O2 + 90% Ar | 5.0 × 10−3 | 30 |
| 1000 | 100 | 10% O2 + 90% Ar | 5.0 × 10−3 | 30 |
| 1000 | 200 | 10% O2 + 90% Ar | 5.0 × 10−3 | 30 |
| Samples | Contact Angle [°] | |
|---|---|---|
| Water | Diodomethane | |
| Non-coated | 98.8 ± 2.4 | 38.8 ± 5.0 |
| 650 W Ti/15 W Cu | 85.1 ± 3.1 | 40.8 ± 3.1 |
| 650 W Ti/100 W Cu | 85.5 ± 2.5 | 41.9 ± 3.3 |
| 650 W Ti/200 W Cu | 80.4 ± 2.2 | 36.4 ± 2.8 |
| 1000 W Ti/15 W Cu | 56.2 ± 3.6 | 31.3 ± 2.8 |
| 1000 W Ti/100 W Cu | 68.2 ± 2.5 | 37.2 ± 3.1 |
| 1000 W Ti/200 W Cu | 63.4 ± 2.3 | 31.4 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P.; Woskowicz, E.; Życki, M. Influence of Deposition Parameters of TiO2 + CuO Coating on the Membranes Surface Used in the Filtration Process of Dairy Wastewater on Their Functional Properties. Membranes 2021, 11, 290. https://doi.org/10.3390/membranes11040290
Kacprzyńska-Gołacka J, Łożyńska M, Barszcz W, Sowa S, Wieciński P, Woskowicz E, Życki M. Influence of Deposition Parameters of TiO2 + CuO Coating on the Membranes Surface Used in the Filtration Process of Dairy Wastewater on Their Functional Properties. Membranes. 2021; 11(4):290. https://doi.org/10.3390/membranes11040290
Chicago/Turabian StyleKacprzyńska-Gołacka, Joanna, Monika Łożyńska, Wioletta Barszcz, Sylwia Sowa, Piotr Wieciński, Ewa Woskowicz, and Maciej Życki. 2021. "Influence of Deposition Parameters of TiO2 + CuO Coating on the Membranes Surface Used in the Filtration Process of Dairy Wastewater on Their Functional Properties" Membranes 11, no. 4: 290. https://doi.org/10.3390/membranes11040290
APA StyleKacprzyńska-Gołacka, J., Łożyńska, M., Barszcz, W., Sowa, S., Wieciński, P., Woskowicz, E., & Życki, M. (2021). Influence of Deposition Parameters of TiO2 + CuO Coating on the Membranes Surface Used in the Filtration Process of Dairy Wastewater on Their Functional Properties. Membranes, 11(4), 290. https://doi.org/10.3390/membranes11040290