A Review on Mixed Matrix Membranes for Solvent Dehydration and Recovery Process
Abstract
:1. Introduction
Solvent Dehydration and Solvent Recovery
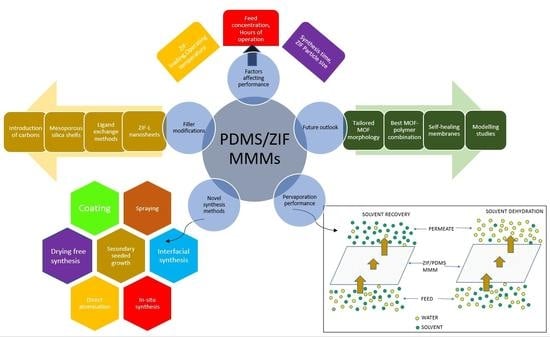
2. ZIF/PDMS Membranes
2.1. Challenges in the Fabrication of MMMs
2.2. Superhydrophobic Membranes
2.3. Modified ZIF Particles
2.4. Modified Synthesis Procedures
2.5. Modelling Studies on MMMs
2.6. Separation Process Involved
3. Factors Affecting Pervaporation Performance of ZIF-PDMS Membranes
3.1. ZIF Loading
3.2. Operating Temperature
3.3. Feed Concentration
3.4. Hours of Operation
3.5. Synthesis Time
3.6. ZIF Particle Size
3.7. Membrane Thickness
4. ZIF-Polymer MMMs Involving Other Polymers
5. Transport Mechanism
Maxwell Model
6. Conclusions
7. Future Outlook
- (1)
- Ways to tailor the morphology and functional groups of the MOFs to improve MOF-polymer interactions. The morphology of the fillers can be changed from spherical to lamellar or fibrous-shaped, for better performance. Functional groups can be changed to make the MOFs dynamic/responsive to external stimuli like temperature and pressure [18]. Low molecular weight coupling agents can also be used for better polymer-MOF interactions [19]. Methods of priming and thermal annealing can also be used [84].
- (2)
- Process of tuning the micro-structure of the filler particles, keeping in mind the crystallinity and fractional free volume. One of the methods to do this is to make the pore orientation parallel to the direction of gas diffusion. However, it is difficult to achieve and a method is required for the growth of the particles along with the desired pore orientation [18].
- (3)
- Methods to select the best MOF-polymer combination and studying the effect of the MOF on the corresponding polymer. For instance, choosing a polymer that allows for a better porous structure when combined with the inorganic fillers can help improve separation [18].
- (4)
- Finding more stable and chemically resistant membrane materials. Generally, MOFs are vulnerable to acidic conditions. For instance, nano-sized fillers resistant to aggregation and harsh chemical conditions should be looked into [18].
- (5)
- Performing modelling studies to predict MMM performance. There are only a few studies that take into account the presence of interface defects and their effect on membrane performance. Such studies might be difficult to perform experimentally, and, thus, need molecular simulations as the role of the interface is important in the diffusion through the membrane [85]. Modelling studies can also help identify compatible MOF-polymer pairs as well as ways to improve the compatibility for the pairs that are not [86].
- (6)
- Optimization of the membrane structure to reduce mass-transfer resistance [26]. This can be done through modelling studies.
- (7)
- Exploring the concept of self-healing for membranes used in solvent separation and dehydration. For instance, a self-healing membrane has been developed for the separation of oil from wastewater using a ZIF-PDMS membrane modified with multi-walled carbon nanotube film. Such a membrane was able to handle harsh environmental conditions and perform self-healing upon external damage [87].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajawat, A.; Sundarrajan, S.; Ramakrishna, S. Progress on Silica Pervaporation Membranes in Solvent Dehydration and Solvent Recovery Processes. Materials 2020, 13, 3354. [Google Scholar] [CrossRef]
- Ruthusree, S.; Sundarrajan, S.; Ramakrishna, S. Progress and Perspectives on Ceramic Membranes for Solvent Recovery. Membranes 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Rev. Chem. Eng. 2019, 35, 565–590. [Google Scholar] [CrossRef]
- Ying, Y.; Xiao, Y.; Yang, Q.; Liu, D.; Ma, J.; Guo, X.; Huang, H.; Zhong, C. Recovery of acetone from aqueous solution by ZIF-7/PDMS mixed matrix membranes. RSC Adv. 2015, 5, 28394–28400. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Valdez, J. New Trends in Biopolymer-Based Membranes for Pervaporation. Molecules 2019, 24, 3584. [Google Scholar] [CrossRef] [Green Version]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Saw, E.T.; Ang, K.L.; He, W.; Dong, X.; Ramakrishna, S. Molecular sieve ceramic pervaporation membranes in solvent recovery: A comprehensive review. J. Environ. Chem. Eng. 2019, 7, 103367. [Google Scholar] [CrossRef]
- Urtiaga, A.M.; Gorri, E.D.; Gomez, P.; Casado, C.; Ibañez, R.; Ortiz, I.; Gorri, D. Pervaporation Technology for the Dehydration of Solvents and Raw Materials in the Process Industry. Dry. Technol. 2007, 25, 1819–1828. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, X. Preparation of Zeolitic Imidazolate Framework-91 and its modeling for pervaporation separation of water/ethanol mixtures. Sep. Purif. Technol. 2020, 237, 116330. [Google Scholar] [CrossRef]
- Gallego-Lizon, T.; Edwards, E.; LoBiundo, G.; Dos Santos, L.F. Dehydration of water/t-butanol mixtures by pervaporation: Comparative study of commercially available polymeric, microporous silica and zeolite membranes. J. Membr. Sci. 2002, 197, 309–319. [Google Scholar] [CrossRef]
- Yin, H.; Lau, C.Y.; Rozowski, M.; Howard, C.; Xu, Y.; Lai, T.; Dose, M.E.; Lively, R.P.; Lind, M.L. Free-standing ZIF-71/PDMS nanocomposite membranes for the recovery of ethanol and 1-butanol from water through pervaporation. J. Membr. Sci. 2017, 529, 286–292. [Google Scholar] [CrossRef]
- Si, Z.; Cai, D.; Li, S.; Zhang, C.; Qin, P.; Tan, T. Carbonized ZIF-8 incorporated mixed matrix membrane for stable ABE recovery from fermentation broth. J. Membr. Sci. 2019, 579, 309–317. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Zhao, X.; Jin, W. Hydrophobic-ZIF-71 filled PEBA mixed matrix membranes for recovery of biobutanol via pervaporation. J. Membr. Sci. 2013, 446, 181–188. [Google Scholar] [CrossRef]
- Si, Z.; Cai, D.; Li, S.; Li, G.; Wang, Z.; Qin, P. A high-efficiency diffusion process in carbonized ZIF-8 incorporated mixed matrix membrane for n-butanol recovery. Sep. Purif. Technol. 2019, 221, 286–293. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 1–33. [Google Scholar] [CrossRef]
- Budiyono, B.; Joko, K.T.; Seno, J.; Sunarso, S. Synthesis and Characterization of Polyimidezeolite Mixed Matrix Membrane for Biogas Purification. Reaktor 2009, 12, 245–252. [Google Scholar]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Shimekit, B.; Shariff, A.M.; Mukhtar, H.; Bustam, M.A.; Elkhalifah, A.E.; Ullah, S.; Riaz, N.; Bustam, A. Interfacial Defects on Mixed Matrix Membranes and Mitigation Techniques for Gas Separation: A Review. Appl. Mech. Mater. 2014, 625, 653–656. [Google Scholar] [CrossRef]
- Chen, F.; Dong, S.; Wang, Z.; Xu, J.; Xu, R.; Wang, J. Preparation of mixed matrix composite membrane for hydrogen purification by incorporating ZIF-8 nanoparticles modified with tannic acid. Int. J. Hydrog. Energy 2020, 45, 7444–7454. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Yu, F.; Zhao, X.; Wang, Y. Enhanced ethanol recovery of PDMS mixed matrix membranes with hydrophobically modified ZIF-90. Sep. Purif. Technol. 2018, 206, 80–89. [Google Scholar] [CrossRef]
- Naik, P.V.; Wee, L.H.; Meledina, M.; Turner, S.; Li, Y.; Van Tendeloo, G.; Martens, J.A.; Vankelecom, I.F.J. PDMS membranes containing ZIF-coated mesoporous silica spheres for efficient ethanol recovery: Via pervaporation. J. Mater. Chem. A 2016, 4, 12790–12798. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, H.; Xu, S.; Wang, Y. ZIF-8 membrane synthesized via covalent-assisted seeding on polyimide substrate for pervaporation dehydration. AIChE J. 2019, 65. [Google Scholar] [CrossRef]
- Yao, J.; Wang, H. Zeolitic imidazolate framework composite membranes and thin films: Synthesis and applications. Chem. Soc. Rev. 2014, 43, 4470–4493. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, C.; Yang, F.; Xiang, L.; Huang, X.; Yu, J.; Zhang, L.; Pan, Y.; Li, Y. Enhanced C3H6/C3H8 separation performance on MOF membranes through blocking defects and hindering framework flexibility by silicone rubber coating. Chem. Commun. 2017, 53, 7760–7763. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Barankova, E.; Pradeep, N.; Peinemann, K.-V. Zeolite-imidazolate framework (ZIF-8) membrane synthesis on a mixed-matrix substrate. Chem. Commun. 2013, 49, 9419–9421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wee, L.H.; Volodin, A.; Martens, J.; Vankelecom, I.F.J. Polymer supported ZIF-8 membranes prepared via an interfacial synthesis method. Chem. Commun. 2014, 51, 918–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amooghin, A.E.; Mashhadikhan, S.; Sanaeepur, H.; Moghadassi, A.; Matsuura, T.; Ramakrishna, S. Substantial breakthroughs on function-led design of advanced materials used in mixed matrix membranes (MMMs): A new horizon for efficient CO2 separation. Prog. Mater. Sci. 2019, 102, 222–295. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Q.; Shen, J.; Huang, K.; Liu, G.; Zhao, J.; Duan, J.; Jin, W. Hydrophobic-functionalized ZIF-8 nanoparticles incorporated PDMS membranes for high-selective separation of propane/nitrogen. Asia Pac. J. Chem. Eng. 2017, 12, 110–120. [Google Scholar] [CrossRef]
- Fan, H.; Wang, N.; Ji, S.; Yan, H.; Zhang, G. Nanodisperse ZIF-8/PDMS hybrid membranes for biobutanol permselective pervaporation. J. Mater. Chem. A 2014, 2, 20947–20957. [Google Scholar] [CrossRef]
- Jin, M.-Y.; Lin, Y.; Liao, Y.; Tan, C.-H.; Wang, R. Development of highly-efficient ZIF-8@PDMS/PVDF nanofibrous composite membrane for phenol removal in aqueous-aqueous membrane extractive process. J. Membr. Sci. 2018, 568, 121–133. [Google Scholar] [CrossRef]
- Fan, H.; Shan, L.; Meng, H.; Zhang, G. High-throughput production of nanodisperse hybrid membranes on various substrates. J. Membr. Sci. 2018, 552, 177–188. [Google Scholar] [CrossRef]
- Li, J.; Wang, N.; Yan, H.; Ji, S.; Zhang, G. Designing superhydrophobic surfaces with SAM modification on hierarchical ZIF-8/polymer hybrid membranes for efficient bioalcohol pervaporation. RSC Adv. 2014, 4, 59750–59753. [Google Scholar] [CrossRef]
- Yuan, S.; Zhu, J.; Li, Y.; Zhao, Y.; Li, J.; Van Puyvelde, P.; Van der Bruggen, B. Structure architecture of micro/nanoscale ZIF-L on a 3D printed membrane for a superhydrophobic and underwater superoleophobic surface. J. Mater. Chem. A 2019, 7, 2723–2729. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Yang, Y.; Si, Z.; Li, P.; Qin, P.; Tan, T. Improving the pervaporation performance of PDMS membranes for n-butanol by incorporating silane-modified ZIF-8 particles. Sep. Purif. Technol. 2019, 215, 163–172. [Google Scholar] [CrossRef]
- Wang, N.; Shi, G.; Gao, J.; Li, J.; Wang, L.; Guo, H.; Zhang, G.; Ji, S. MCM-41@ZIF-8/PDMS hybrid membranes with micro- and nanoscaled hierarchical structure for alcohol permselective pervaporation. Sep. Purif. Technol. 2015, 153, 146–155. [Google Scholar] [CrossRef]
- Mao, H.; Zhen, H.-G.; Ahmad, A.; Li, S.-H.; Liang, Y.; Ding, J.-F.; Wu, Y.; Li, L.-Z.; Zhao, Z.-P. Highly selective and robust PDMS mixed matrix membranes by embedding two-dimensional ZIF-L for alcohol permselective pervaporation. J. Membr. Sci. 2019, 582, 307–321. [Google Scholar] [CrossRef]
- Yin, H.; Cay-Durgun, P.; Lai, T.; Zhu, G.; Engebretson, K.; Setiadji, R.; Green, M.D.; Lind, M.L. Effect of ZIF-71 ligand-exchange surface modification on biofuel recovery through pervaporation. Polymer 2020, 195, 122379. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.-H.; Zhang, A.-S.; Xu, L.-H.; Lu, J.-J.; Zhao, Z.-P. Novel MOF-capped halloysite nanotubes/PDMS mixed matrix membranes for enhanced n-butanol permselective pervaporation. J. Membr. Sci. 2020, 595, 117543. [Google Scholar] [CrossRef]
- Zhu, T.; Xu, S.; Yu, F.; Yu, X.; Wang, Y. ZIF-8@GO composites incorporated polydimethylsiloxane membrane with prominent separation performance for ethanol recovery. J. Membr. Sci. 2020, 598. [Google Scholar] [CrossRef]
- Mao, H.; Zhen, H.-G.; Ahmad, A.; Zhang, A.-S.; Zhao, Z.-P. In situ fabrication of MOF nanoparticles in PDMS membrane via interfacial synthesis for enhanced ethanol permselective pervaporation. J. Membr. Sci. 2019, 573, 344–358. [Google Scholar] [CrossRef]
- Li, G.; Si, Z.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. The in-situ synthesis of a high-flux ZIF-8/polydimethylsiloxane mixed matrix membrane for n-butanol pervaporation. Sep. Purif. Technol. 2020, 236, 116263. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, X.; Yi, M.; Wang, Y. Facile Covalent Crosslinking of Zeolitic Imidazolate Framework/Polydimethylsiloxane Mixed Matrix Membrane for Enhanced Ethanol/Water Separation Performance. ACS Sustain. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Sun, T.; Fang, M.; Wu, Z.; Yu, L.; Li, J. Molecular dynamics insights into the structural and diffusive properties of ZIF-8/PDMS mixedmatrix membranes in n-butanol/water pervaporation process. Mater. Sci. Eng. 2017, 25, 035002. [Google Scholar]
- Singh, T.; Kang, D.-Y.; Nair, S. Rigorous calculations of permeation in mixed-matrix membranes: Evaluation of interfacial equilibrium effects and permeability-based models. J. Membr. Sci. 2013, 448, 160–169. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Kansara, A.M.; Aswal, V.K.; Singh, P.S. Effect of Zeolitic Imidazole Framework-8 nanocrystals on hydrocarbon permselective Poly(dimethylsiloxane) membrane as probed by small-angle neutron scattering. Polymer 2018, 143, 96–105. [Google Scholar] [CrossRef]
- Fan, H.; Shi, Q.; Yan, H.; Ji, S.; Dong, J.; Zhang, G. Simultaneous Spray Self-Assembly of Highly Loaded ZIF-8-PDMS Nanohybrid Membranes Exhibiting Exceptionally High Biobutanol-Permselective Pervaporation. Angew. Chem. Int. Ed. 2014, 53, 5578–5582. [Google Scholar] [CrossRef] [PubMed]
- Žák, M.; Klepic, M.; Štastná, L.Č.; Sedláková, Z.; Vychodilová, H.; Hovorka, Š.; Friess, K.; Randová, A.; Brožová, L.; Jansen, J.C.; et al. Selective removal of butanol from aqueous solution by pervaporation with a PIM-1 membrane and membrane aging. Sep. Purif. Technol. 2015, 151, 108–114. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, G.; Jin, W. Recent Progress in Separation Membranes and Their Fermentation Coupled Processes for Biobutanol Recovery. Energy Fuels 2020, 34, 11962–11975. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Fang, M.; Wang, T.; Yu, L.; Li, J. ZIF-7/PDMS mixed matrix membranes for pervaporation recovery of butanol from aqueous solution. Sep. Purif. Technol. 2016, 163, 39–47. [Google Scholar] [CrossRef]
- Yin, H.; Khosravi, A.; O’Connor, L.; Tagaban, A.Q.; Wilson, L.; Houck, B.; Liu, Q.; Lind, M.L. Effect of ZIF-71 Particle Size on Free-Standing ZIF-71/PDMS Composite Membrane Performances for Ethanol and 1-Butanol Removal from Water through Pervaporation. Ind. Eng. Chem. Res. 2017, 56, 9167–9176. [Google Scholar] [CrossRef]
- Su, P.; Zhang, X.; Li, Y.; Chen, H.; Meng, Q.; Zhang, G. Distillation of alcohol/water solution in hybrid metal–organic framework hollow fibers. AIChE J. 2019, 65. [Google Scholar] [CrossRef]
- Fang, M.; Wu, C.; Yang, Z.; Wang, T.; Xia, Y.; Li, J. ZIF-8/PDMS mixed matrix membranes for propane/nitrogen mixture separation: Experimental result and permeation model validation. J. Membr. Sci. 2015, 474, 103–113. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, L.; Zhang, C.; Gu, J.; Sun, Y.; Zhang, L.; Chen, H. ZIF-8 Filled Polydimethylsiloxane Membranes for Pervaporative Separation ofn-Butanol from Aqueous Solution. Sep. Sci. Technol. 2013, 48, 2531–2539. [Google Scholar] [CrossRef]
- Khan, A.; Ali, M.; Ilyas, A.; Naik, P.; Vankelecom, I.F.; Gilani, M.A.; Bilad, M.R.; Sajjad, Z.; Khan, A.L. ZIF-67 filled PDMS mixed matrix membranes for recovery of ethanol via pervaporation. Sep. Purif. Technol. 2018, 206, 50–58. [Google Scholar] [CrossRef]
- Rao, Y.; Ni, F.; Sun, Y.; Zhu, B.; Zhou, Z.; Yao, Z. Efficient recovery of the volatile aroma components from blackberry juice using a ZIF-8/PDMS hybrid membrane. Sep. Purif. Technol. 2020, 230, 115844. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, L.; Shen, J.; Shi, J.; Chen, G.; Zhao, J.; Duan, J.; Liu, G.; Jin, W. Improved ethanol recovery through mixed-matrix membrane with hydrophobic MAF-6 as filler. Sep. Purif. Technol. 2017, 178, 105–112. [Google Scholar] [CrossRef]
- Dobrak, A.; Figoli, A.; Chovau, S.; Galiano, F.; Simone, S.; Vankelecom, I.; Drioli, E.; Van der Bruggen, B. Performance of PDMS membranes in pervaporation: Effect of silicalite fillers and comparison with SBS membranes. J. Colloid Interface Sci. 2010, 346, 254–264. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Martens, J.A.; Vankelecom, I.F.J. ZIF-71 as a potential filler to prepare pervaporation membranes for bio-alcohol recovery. J. Mater. Chem. A 2014, 2, 10034–10040. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Guo, Y.; Wang, X.; Li, P.; Ying, W.; Chen, D.; Ma, X.; Deng, Z.; Peng, X. Simultaneous Recovery of Metal Ions and Electricity Harvesting via K-Carrageenan@ZIF-8 Membrane. ACS Appl. Mater. Interfaces 2019, 11, 34039–34045. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y. Poly(vinyl alcohol)/ZIF-8-NH2mixed matrix membranes for ethanol dehydration via pervaporation. AIChE J. 2016, 62, 1728–1739. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Chen, J.-T.; Chang, C.-H.; Liao, K.-S.; Tung, K.-L.; Price, W.E.; Yamauchi, Y.; Wu, K.C.-W. A Drying-Free, Water-Based Process for Fabricating Mixed-Matrix Membranes with Outstanding Pervaporation Performance. Angew. Chem. 2016, 128, 12985–12988. [Google Scholar] [CrossRef]
- Fazlifard, S.; Mohammadi, T.; Bakhtiari, O. Chitosan/ZIF-8 Mixed-Matrix Membranes for Pervaporation Dehydration of Isopropanol. Chem. Eng. Technol. 2017, 40, 648–655. [Google Scholar] [CrossRef]
- Kang, C.-H.; Lin, Y.-F.; Huang, Y.-S.; Tung, K.-L.; Chang, K.-S.; Chen, J.-T.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Synthesis of ZIF-7/chitosan mixed-matrix membranes with improved separation performance of water/ethanol mixtures. J. Membr. Sci. 2013, 438, 105–111. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, X.; Li, C.; Hao, X.; Wang, Y.; Guan, G. ZIF-8 incorporated polyether block amide membrane for phenol permselective pervaporation with high efficiency. Sep. Purif. Technol. 2016, 166, 252–261. [Google Scholar] [CrossRef]
- Flyagina, I.S.; Mahdi, E.M.; Titov, K.; Tan, J.-C. Thermo-mechanical properties of mixed-matrix membranes encompassing zeolitic imidazolate framework-90 and polyvinylidine difluoride: ZIF-90/PVDF nanocomposites. APL Mater. 2017, 5, 086104. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415–416, 577–586. [Google Scholar] [CrossRef]
- Benzaqui, M.; Semino, R.; Carn, F.; Tavares, S.R.; Menguy, N.; Giménez-Marqués, M.; Bellido, E.; Horcajada, P.; Berthelot, T.; Kuzminova, A.I.; et al. Covalent and Selective Grafting of Polyethylene Glycol Brushes at the Surface of ZIF-8 for the Processing of Membranes for Pervaporation. ACS Sustain. Chem. Eng. 2019, 7, 6629–6639. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Hu, P.; Peng, X. ZIF-8 coated polyvinylidenefluoride (PVDF) hollow fiber for highly efficient separation of small dye molecules. Appl. Mater. Today 2016, 5, 103–110. [Google Scholar] [CrossRef]
- Cheong, F.; Suzanna, R.W.; Lim, K.P.; Ng, W.F.; Moh, P.Y. GO@ZIF-67/PAN Mixed Matrix Membrane for the Adsorptive and Photocatalytic Removal of Methylene Blue. Trans. Sci. Technol. 2017, 4, 202–208. [Google Scholar]
- Li, W.; Li, J.; Wang, N.; Li, X.; Zhang, Y.; Ye, Q.; Ji, S.; An, Q.-F. Recovery of bio-butanol from aqueous solution with ZIF-8 modified graphene oxide composite membrane. J. Membr. Sci. 2020, 598, 117671. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Q.; Wu, C.; Wang, H.; Wang, H. Viscosity-driven in situ self-assembly strategy to fabricate cross-linked ZIF-90/PVA hybrid membranes for ethanol dehydration via pervaporation. Sep. Purif. Technol. 2018, 201, 256–267. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Shi, W.; Zhang, H.; Zhou, R.; Qin, X. Preparation of hydrophobic zeolitic imidazolate framework-71 (ZIF-71)/PVDF hollow fiber composite membrane for membrane distillation through dilute solution coating. Sep. Purif. Technol. 2020, 251, 117348. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Liu, J.; He, J.; Li, J.; Wang, L.; Lei, J. Fabrication and characterization of a defect-free mixed matrix membrane by facile mixing PPSU with ZIF-8 core–shell microspheres for solvent-resistant nanofiltration. J. Membr. Sci. 2019, 589, 117261. [Google Scholar] [CrossRef]
- Low, Z.-X.; Razmjou, A.; Wang, K.; Gray, S.; Duke, M.; Wang, H. Effect of addition of two-dimensional ZIF-L nanoflakes on the properties of polyethersulfone ultrafiltration membrane. J. Membr. Sci. 2014, 460, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Hu, T.; Wang, Y.; Chen, H.; Wang, Y.; Yao, R.; Ma, X.; Li, J.; Li, X. A water-based mixing process for fabricating ZIF-8/PEG mixed matrix membranes with efficient desulfurization performance. Sep. Purif. Technol. 2019, 214, 61–66. [Google Scholar] [CrossRef]
- Wang, Z.; Si, Z.; Cai, D.; Li, G.; Li, S.; Qin, P.; Tan, T. Improving ZIF-8 stability in the preparation process of polyimide-based organic solvent nanofiltration membrane. Sep. Purif. Technol. 2019, 227, 115687. [Google Scholar] [CrossRef]
- Xu, S.-J.; Shen, Q.; Chen, G.-E.; Xu, Z.-L. Novel β-CD@ZIF-8 Nanoparticles-Doped Poly(m-phenylene isophthalamide) (PMIA) Thin-Film Nanocomposite (TFN) Membrane for Organic Solvent Nanofiltration (OSN). ACS Omega 2018, 3, 11770–11787. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Pan, F.; Li, Y.; Quan, K.; Jiang, Z. Mass transport mechanisms within pervaporation membranes. Front. Chem. Sci. Eng. 2019, 13, 458–474. [Google Scholar] [CrossRef]
- Monsalve-Bravo, G.M.; Bhatia, S.K. Modeling Permeation through Mixed-Matrix Membranes: A Review. Processes 2018, 6, 172. [Google Scholar] [CrossRef] [Green Version]
- Bánhegyi, G. Comparison of electrical mixture rules for composites. Colloid Polym. Sci. 1986, 264, 1030–1050. [Google Scholar] [CrossRef]
- Jeffrey, D.J. Conduction through a random suspension of spheres. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1973, 335, 355–367. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Chew, T.L.; Lau, K.K.; Shariff, A.M. Current Development and Challenges of Mixed Matrix Membranes for CO2/CH4Separation. Sep. Purif. Rev. 2016, 45, 321–344. [Google Scholar] [CrossRef]
- Ozcan, A.; Semino, R.; Maurin, G.; Yazaydin, A.O. Modeling of Gas Transport through Polymer/MOF Interfaces: A Microsecond-Scale Concentration Gradient-Driven Molecular Dynamics Study. Chem. Mater. 2020, 32, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Dutta, R.C.; Bhatia, S.K. Interfacial Engineering of MOF-Based Mixed Matrix Membrane through Atomistic Simulations. J. Phys. Chem. C 2019, 124, 594–604. [Google Scholar] [CrossRef]
- Ye, H.; Chen, D.; Li, N.-J.; Xu, Q.-F.; Li, H.; He, J.-H.; Lu, J.-M. Durable and Robust Self-Healing Superhydrophobic Co-PDMS@ZIF-8-Coated MWCNT Films for Extremely Efficient Emulsion Separation. ACS Appl. Mater. Interfaces 2019, 11, 38313–38320. [Google Scholar] [CrossRef] [PubMed]









| Sl.no. | ZIF Type | PDMS ZIF/PDMS/Solvent | MW | Blending Conditions | Size of ZIF Particles/MMM Thickness,% of ZIF Loading | Solvent Systems (S.F.-Separation Factor) | References |
|---|---|---|---|---|---|---|---|
| 1. | ZIF-7 Superhydrophobic | Heptane | 10,000 g/mol PDMS | ZIF-7 in n-heptane with stirring for 6 h. PDMS added and stirred for 4 h. Tetraethyl orthosilicate (TEOS) and dibutyltin dilaurate (DBTDL) added. PDMS:TEOS:DBTDL = 10:1:0.1. Polyvinylidene difluoride (PVDF) film used as the support. Membrane dried in a vacuum oven for 8 h. | 80 nm sized particles 0–40 wt% loading | Acetone/water at 333 K (40 wt% loading) Flux-1542.6 g/m2 h (25 wt% loading) Flux-1236.8 g/m2 h SF-39.1 | [5] |
| 2. | ZIF-90 ZIF-91 ZIF-92 | Tetrahydrofuran (THF) | 3000 mPa.s viscous PDMS | ZIF added into PDMS/THF solution and stirred vigorously for 24 h. Curing agent (TEOS) and catalyst DBTDL added under magnetic stirring at room temperature for 2 h. Solution degassed and applied on the PVDF substrate with a casting knife. Dried overnight at 60 °C on vacuum condition, followed by annealing of fabricated MMMs at 80 °C for 2 h. | Average diameters: 132 nm (ZIF-90), 275 nm (ZIF-91) and 1208 nm (ZIF-92) 20 wt% loading | 5 wt% ethanol-water solution at 55 °C Best:ZIF-91/PDMS Flux-846 g/m2 h SF-15.8 | [10] |
| 3. | ZIF-71 Hydrophobic | Heptane | 110,000 g/mol PDMS | PDMS-heptane solution. ZIF-71-heptane solution. Two solutions mixed. TEOS added. Titanium 2-ethylhexoxide and di-n-butyldiacetoxytin tech-95 added. Film poured on Teflon flat dish and dried. | 646.2 ± 6.3 nm sized particles 100–300 µm thick membrane 40 wt% loading | 1-butanol/water SF-69.9 ± 1.8 Ethanol/water SF-9.2 ± 0.7 | [12] |
| 4. | ZIF-8 Carbonized-hydrophobic | Hexane ZNC/PDMS | 5000 mPa s Viscous PDMS | PDMS+ZNC particles+TEOS mixed in n-hexane. DBTDL added and the mixture stirred and degassed. Casting solution cast on a piece of polyvinylidene fluoride (PVDF) membrane. | 9.53 μm to 11.96 μm thick membrane 10 wt% loading | ABE fermentation brothFlux-1870 g/m2 h SF- 16.8 (acetone) 4.5 (ethanol) 20.7 (n-butanol) | [13] |
| 5. | ZIF-8 Carbonized | ZNC/PDMS Hexane | 5000 mPa s Viscous PDMS | PDMS+ZNC particles+ TEOS mixed in n-hexane and stirred for 2 h. PDMS and TEOS was 18:1. DBTDL added and the mixture stirred for 5 min and degassed. Casting solution cast on a piece of polyvinylidene fluoride (PVDF) membrane. | 3 wt% loading | 1.5 wt% butanol/water solution at 55 °C Flux-1249.5 g/m2 h SF-53.1 | [15] |
| 6. | ZIF-90 dodecylamine-modified ((DLA-ZIF-90) Hydrophobic | Hexane | NA | PVDF membrane, fabricated by the non-solvent induced phase inversion process, was used as the support for the preparation of PDMS MMMs. Particles added to PDMS/hexane solution with 12 h vigorous stirring and 2 h sonication. 10 wt% curing agent added at 60 °C with 2 h stirring. Overnight degassing, casting on PVDF support and drying at 60 °C under vacuum overnight. Annealed at 100 °C for another 6 h | 500 nm sized particles Thickness: 107 µm (1 wt% loading), 100 µm (2.5 wt% loading), 125 µm (5 wt% loading) 1, 2.5 and 5 wt% loadings Optimum- 2.5 wt% loading | Ethanol recovery at 60 °C 5/95 wt% ethanol/water mixture 2.5 wt% loading: Flux-99.5 g/m2 h SF-15.1 | [21] |
| 7. | MSS-ZIF-71 and MSS-ZIF-8 (mesoporous silica spheres) | Hexane | NA | Two components of the PDMS (RTV-615 A and B, prepolymer and cross-linker, respectively) dissolved separately in hexane. The MSS–ZIF nanoparticles dispersed ultrasonically in hexane for 1 h. Prepolymer, the cross-linker and the filler mixed and stirred at 60 °C for 4 h. Solution poured into a glass petri dish, kept in an oven at 110 °C for at least 1 h. | 2–3 µm sized particles 10 µm thick membrane 10, 15, 20 wt% loading | 6 wt% Ethanol/water (with 20 wt% loading) MSS-ZIF-71: Flux-1000 g/m2 h SF-13 MSS-ZIF-8: Flux-720 g/m2 h SF-15 | [22] |
| 8. | ZIF-8 | Hexane PDMS–ZIF/PI–ICA | NA | Polyimide substrate prepared and washed with methanol and soaked with 10 wt% ethylenediamine methanol solution at room temperature for 10 min followed by immersion in 1 wt% ICA methanol solution at 60 °C for 5 min followed by horizontal immersion in Zn(CH3COO)2·2H2O solution and 1 min sonication. Hmim and ammonia hydroxide solutions added. Ultrasonication for 5 min. Overnight crystallisation and washing with water. Membrane soaked in secondary growth solution (mixture of Zn(NO3)2·6H2O and Hmim solution) for 6 hr at room temp, washed and soaked in PDMS solution (5 wt% in hexane) for 3 min. | 40–50 nm sized particles 190.4 nm thick skin layer | Isopropanol dehydration 85/15 wt% IPA/water mixture at 40 °C | [23] |
| 9. | ZIF-8 | Heptane 10 wt% PDMS | 20,000 Pa s Viscous PDMS | Polysulfone (PS) ultrafiltration sheet supports. Non-dried ZIF-8/ethanol suspensions dispersed dropwise in dilute PDMS/n-heptane solution (labeled as suspension-dispersed ZIF-8/PDMS). TEOS (1 wt%) and DBTDL (0.05 wt%) added and stirred for 0.5 h. A concentrated PDMS pre-cross-linked solution (10 wt%) prepared. First, a homogeneous ZIF8/PDMS membrane formed by repeatedly and horizontally dipping the pre-treated PS supporting membrane in the ZIF-8/PDMS solution (1 wt%) (1 min immersion per layer, 30 s intervals), followed by fixing perpendicularly onto a substrate with a rotating motor with continuous baking by a burner to remove the residual solution on the support surface. Membrane dipped once in the concentrated PDMS. Membranes allowed to stand for 1 day in air at room temperature, then placed in a convection oven at 80 °C for 8 h. | 1 µm sized particles 1.8 µm thick membrane | 5.0 wt% n-butanol–water solution at 80 °C Flux-2500.8 g/m2 hSF-52.81 | [31] |
| 10. | ZIF-8 | ZIF-8@PDMS/PVDF nanofibrous composite membrane Hexane 10 wt% PDMS | 18,000–22,000 cSt viscous PDMS | Drying free process: PDMS/hexane solution prepared. ZIF/hexane solution prepared. PDMS solution added dropwise followed by vigorous stirring for 1 h. Cross-linker TEOS (5 wt%) and catalyst DBTDL (1 wt%) added. Solution poured into an aluminium petri dish. Complete solvent evaporation at room temperature followed by heat cured in an oven at 80 °C for 24 h. Electrospun PVDF nanofibrous membrane used as support. | 180 nm sized particles ZIF-8@PDMS: 400 ± 30 µm thick membranes ZIF-8@PDMS/PVDF: 13.2 ± 1.1 µm thickness 0, 1, 4, 8 wt% loading (4 wt% optimum) | Phenol separation in the aqueous-aqueous membrane extraction process ZIF-8@PDMS: k0 of 2.61 ± 0.05 × 10−7 m/s ZIF-8@PDMS/PVDF: k0 of 35.7 ± 1.1 × 10−7 m/s | [32] |
| 11. | ZIF-8 | No solvent | 300 mPa·s, viscous and 14,000 g/mol PDMS | Polymethylhydrosiloxane (PMHS) (cross-linker) and ZIF dissolved in ethanol, platinum catalyst added dropwise. PDMS and prepared suspension repeatedly and alternately sprayed onto a rotating Polysulfone membrane surface using two horizontal spray nozzles at 80 °C | 90 nm sized particles 6 µm thick selective layer 0–15 wt% loading | 1–5 wt% aqueous butanol solution at 30–70 °C Flux-2334.6 g/m2 h SF-64.5 | [33] |
| 12. | ZIF-8 | ZIF/PDMS ZIF/PDMS/SF | NA | Dip-coating and SAM modification. Membrane stable underwater even after 7 days of immersion. | 80–90 nm sized particles | n-butanol/water (with SAM modification) Flux-1339 g/m2 h SF-84.8 | [34] |
| 13. | ZIF-L | Hexane, Water | NA | 3-D printed PA membrane. Membrane coated with ZIF-L solution and dried for 12 h at 60 C. Resulting membrane coated with Hmim and zinc nitrate solutions to obtain multiscale layers. PDMS solution in n-hexane prepared. Above membrane coated with the solution by immersion for 10 min. Resulting membrane allowed to solidify at 70 °C for 30 min in the oven. | 2.7–7.2 µm sized particles | Oil/water Flux- 24,000 L/m2.h | [35] |
| 14. | Silane modified ZIF-8 P-ZIF-8@PDA and O-ZIF-8@PDA Hydrophobic | Hexane P-ZIF-8@PDA/PDMS and O-ZIF-8@PDA/PDMS | 5000 mPa s viscous PDMS | Particles, PDMS and TEOS added in n-hexane and stirred for 2 h. PDMS:TEOS = 18:1. DBTDL added and stirred for 5 min. Casting solution coated on a PVDF membrane using an automatic film applicator followed by curing at room temperature for 24 h. | 11.5 µm thick membranes 1 wt% loading | 1.5 wt% butanol solution at 55 °C O-ZIF-8 @PDA/PDMS: Flux-480.6 g/m2 h SF-56 P-ZIF-8 @PDA/PDMS: Flux-480.6 g/m2 h SF-47 | [36] |
| 15. | MCM-41@ZIF-8 | Heptane | PDMS kinetic viscosity of 3000 mPa s | Casting solution poured into the surface of PS membrane. The composite membrane was prepared by a doctor blading method. After standing at room temperature for 12 h, the composite membrane was transferred to a vacuum oven at 90 °C for 12 h to fully cross-link | 20–30 nm sized particles 3 µm thick selective layer 5 wt% loading | 5.0 wt% ethanol/water at 70 °C Flux-2204 g/m2 h SF-10.4 3 wt% n-butanol/water at 60 °C Flux-2052 g/m2 h SF-45 | [37] |
| 16. | ZIF-L nanosheets | Heptane | 5000 mPa s viscous PDMS | ZIF-L dispersed in n-heptane using probe sonicator. 10 wt% PDMS added followed by stirring. Cross-linking and binding agent (3-aminopropyl) triethoxysilane (APTES) and catalyst DBTDL added such that 1:0.1:0.05:4 (PDMS: APTES: DBTDL: n-heptane). Solution cast onto the PVDF support. Evaporation of the residual solvent and crosslinked at 120 °C for 3 h. | ZIF-L sheets: dimensions of about 5.6 μm × 2.2 μm and a thickness of about 136 nm. Active layer thickness for each membrane was averaged to be 8.8 μm (10 wt%), 14 μm (20 wt%), 17.3 μm (30 wt%), 23.2 μm (40 wt%), and 38.7 μm (50 wt%) 10,20,30,40,50 wt% loadings | 5 wt% Aqueous alcohol solutions (ethanol, n-propanol or n-butanol) at 40 °C 1.0 wt% n-butanol aqueous solution at 40 °C Flux-402 g/m2 h SF-57.6 | [38] |
| 17. | ZIF-71 | Heptane | 110,000 g/mol PDMS | PDMS/heptane solution added dropwise to ZIF-71-heptane suspension with sonication. After some amount of solvent removal, TEOS added and stirred. TEOS and DBTDL added. Mixture poured into a Teflon flat dish in a humidity-controlled box (75% RH). After 21 h, the films removed and dried in two stages in a vacuum oven; first at 100 °C for 20 h and second in at 120 °C for 11 h. | 506 nm sized particles 25 wt% loading | 2 wt% ethanol/water or 1-butanol/water solution at 60 °C | [39] |
| 18. | ZIF-8 ZIF-8-capped halloysite nanotubes (ZHNT) | Heptane | 50 Pa.s viscous PDMS | ZHNTs dispersed ultrasonically in n-heptane for 1 h. ‘‘Primed’’ by introducing a certain content of PDMS and stirred for 6 h. Remaining PDMS added and stirred for 6 h. TEOS and DBTDL added (PDMS: TEOS: catalyst: n-heptane—1:0.1:0.05:9). Solution cast onto the PVDF substrate. Membranes placed for 15 min at 120 °C for 3 h. | ZHNT: outer diameters of 30–60 nm, inner diameter about 25 nm, length of 400–750 nm The average thickness of separation layer: 10.7 μm (5 wt%), 11.1 μm (10 wt%), 12.2 μm (15 wt%), and 14.8 μm (20 wt%) | 1 wt% n-butanol aqueous solution at 40 °C Flux-683 g/m2 h SF-61.3 | [40] |
| 19. | ZIF-8@GO | THF | 1000 cst viscous PDMS | Doping particles dispersed in THF by sonication and stirring for 12 h. PDMS and TEOS added, followed by sonication for 1 h and stirring for 7 h. Catalyst DBTDL added and stirred for 30 min. After degassing, the solution cast onto the PVDF support layer. Membrane cured at 30 °C for 12 h, and at 80 °C for 6 h. | Less than 50 nm sized particles 9.2 µm thickness 0.75 wt% loading | 5 wt% ethanol aqueous solution at 40 °C Flux-443.8 g/m2 h SF-22.2 | [41] |
| 20. | ZIF-8 | Methanol, ethanol, heptane | 5000 mPa s viscous PDMS | Hmim/methanol/ethanol solution with vigorous stirring. The PDMS/n-heptane solution, cross-linker APTES added, followed by stirring and sonicating alternately for 0.5 h and the catalyst DBTDL was added. After degassing, the resultant organic phase was dip-coated on the PVDF support. Membrane placed in air atmosphere for 10 min and dried sequentially in a 120 °C oven for another 3 h followed by rinsing with methanol and drying. | 1 µm thick active layer | 5.0 wt% ethanol aqueous solution at 40 °C. Flux-1778 g/m2 h SF-12.1 | [42] |
| 21. | ZIF-8 | Hexane | NA | Firstly, Solution A (PDMS/TEOS) and solution B (PDMS/DBTDL) were coated on the PVDF substrate to form the PDMS layer. Then the solution C (PDMS/Zn(NO3)2) and solution D (PDMS/Hmim) were coated on the surface of the PDMS layer in turn. Subsequently, the obtained MMMs were thermally cured at 80 °C for 2 h. The spin-casting procedure operated last for 30 s at 1500 rpm. | Average thickness 17 µm of the active layer 10, 20, 30 wt% loadings | 1.5 wt% n-butanol aqueous solution at 55 °C 20 wt% loading: Flux-2046.3 g/m2 h SF-42.6 | [43] |
| 22. | AZIF-8 (amine-functionalized) | Hexane | Silanol-terminated PDMS (3000 cst, density of 0.98 g/cm3) | ZIF/n-hexane suspension transferred into PDMS/n-hexane solution, followed by vigorous stirring for 5 h, sonication for 1 h, and 2 h stir. Crosslinker GOPTS and catalyst DBTDL added, stirred vigorously for several minutes. After degassing, the mixed solution was cast onto the PVDF support layer, cured at 40 °C for 12 h, and heated to 60 °C for 6 h. | 100 nm sized particles 6.8 µm membrane thickness 7 wt% loading | 5 wt% aqueous ethanol solution at 40 °C Flux-585.6 g/m2 h SF-17.7 | [44] |
| 23. | ZIF-8 | Heptane | NA | ZIF dispersed in n-heptane (ultrasonication with stirring for 2 h), then PDMS (10 wt%) added. Stirring for 1 h. (ZIF:PDMS = 10:60 wt%). Cross-linking agent TEOS (1 wt%)+ catalyst DBTDL (0.05 wt%) dissolved in n-heptane plus stirring at room temp for 1 h. Simultaneous spray self-assembly on sheet polysulfone substrate from two separate barrels with controlled spraying. Vacuum oven at 80 °C for 8 h. | Ultrathin nanohybrid selective layer 800 nm thick top selective layer 10–40% loading | 1 wt% aqueous butanol solution at 80 °C (with 40%loading) Flux-4846.2 g/m2 h SF-81.6 45 wt% of n-butanol recovered | [48] |
| 24. | ZIF-7 | THF | kinetic viscosity, 20,000 mPa s of PDMS | PDMS in THF and stirring for 3 h. ZIF in THF sonicated for 30 min. Both solutions mixed and stirred for 1 h. TEOS and DBTDL added such that 5:1:0.4:20 (PDMS: TEOS: catalyst: THF). Solution poured on the PVDF ultrafiltration membrane and cast with a scraper. Dried overnight and treated at 80 °C for 4 h. | 80 nm sized particles 20 µm thick top selective layer 20 wt% loading | 1 wt% butanol aqueous solution at 60 °C Flux-1689 g/m2 h SF-66 | [51] |
| 25. | ZIF-71 | Heptane | 10,000 g/mol PDMS | Condensation cured membrane. PDMS-heptane solution prepared. ZIF-71-heptane solution prepared with vortex mixing. Both solutions mixed with sonication and vortex mixing. Resulting solution stirred to evaporate the heptane. TEOS and catalyst added. Solution poured on Teflon dish and allowed to dry. | Varying sizes: 152 ± 45 nm, 506 ± 28 nm, and 1030 ± 385 nm 140–390 µm thick membranes 25 wt% loading | 2 wt% 1-butanol/water at 60 °C SF-63 2 wt% 1-ethanol/water at 60 °C SF-12.2 | [52] |
| 26. | ZIF-8 | Heptane | NA | Hybrid hollow fibre membranes. ZIF-heptane solution and PDMS-heptane solution mixed. TEOS and DBTDL added. Mass ratios of ZIF-8 to PDMS set at 10–40%. Resulting solution coated on the inner surface of polyacrylonitrile (PAN) hollow fibres and heated at 60 °C to allow crosslinking. | 100 nm sized particles 5 µm thick membrane 10–40 wt% loading | Isopropanol/water distillation | [53] |
| 27. | ZIF-8 | THF | 50,000 mPa·s viscous PDMS | PDMS-THF solution prepared with stirring for 2 h. ZIF-THF solution prepared with sonication for 20 min in an ice bath followed by warming to room temperature. PDMS solution added to ZIF solution followed by sonication for 10 min. TEOS and catalyst added such that PDMS:TEOS:Catalyst = 100:4:1. Stirring for 2 h followed by casting on PVDF support membrane. | 70 nm sized particles 25 µm thick membrane 0–20 wt% loading (optimum- 10 wt%) | 20%/80% (vol/vol) Propane/nitrogen (For 10 wt% loading) SF-20.5 | [54] |
| 28. | ZIF-8 | DMF | NA | ZIF added to PDMS solution and stirred for 2 h. Solution cast with a knife on a polyethersulfone (PES) ultrafiltration membrane and introduced into a vacuum oven at 80 °C for 20 h for a complete cross-linking reaction. | 50–100 nm sized particles 73 µm thick membrane with 2 wt% loading 1–5 wt% loading (optimum: 2 wt%) | 0.96 wt% n-butanol aqueous solution at 30 °C | [55] |
| 29. | ZIF-67 | Toluene-hexane | NA | Selective layer of PDMS dip-coated on the support. PDMS solution in toluene and hexane (80:20) (RTV 615 A and 615B in a 10:1 ratio) pre-crosslinked for 2 h at 60 °C. Coating solutions loaded with filler concentrations, stirred and sonicated for 15 min each (3 times). The support plate kept at an angle of 60° and PDMS solution poured on the support. This step was repeated at least three times allowing solvents to evaporate for 5 min in between. The cross-linking was completed in an oven at 110 °C for 24 h. | 200 nm sized particles 5 wt%, 10 wt%, 15 wt% and 20 wt% loadings | 6 wt% aqueous ethanol solution at a temperature range of 40–70 °C 20 wt% loading at 40 C: Flux-2780 g/m2 h SF-15.4 | [56] |
| 30. | ZIF-8 | PDMS 20 wt% Tetrahydrofuran | 50,000 mPa·s viscous PDMS | ZIF-8 mixed with THF and stirred for 30 min. PDMS added and stirred for 2 h. Ultrasound performed for 15 min. TEOS and a catalyst added at a mass ratio of 100:4:1. Stirred then allowed to cross-link for 2 h. Solution stirred then poured onto the PVDF membrane. Left at room temperature for 12 h, transferred to a vacuum oven at 130 °C for 2 h. | 60 nm sized particles 3 µm thick membranes 6 wt% loading | Volatile aromatic compounds (VACs) from natural blackberry juice | [57] |
| 31. | ZIF-6 (MAF-6) | Heptane | 60,000 g/mol PDMS | MAF-6/n-heptane suspension stirred and sonicated alternatively for 1/2 h for three times each. Small amount of PDMS added and stirred for 2 h. Suspension mixed with cross-linked PDMS solution (PDMS, n-heptane, TEOS and DBTDL) and stirred for 3–4 h. Membranes cast on PVDF substrate, evaporated at room temperature for 24 h and dried in oven at 70 °C for 12 h. | 150 nm sized particles 5 µm thick membranes 0, 5, 10, 15, 20, 25 wt% loadings | Ethanol/water mixtures at 40 °C 15 wt% loading: Flux-1200 g/m2 h SF-14.9 | [58] |
| Sl.no. | ZIF Type | Polymer/ MMM | Solvent | Blending Conditions | Size Size of ZIF Particles/MMM Thickness, % of ZIF Loading | Solvent Systems (S.F.-Separation Factor) | References |
|---|---|---|---|---|---|---|---|
| 1. | ZIF-8 and ZIF-8-MCM-41 core-shell particles (MSS-Z8) | Polyimide Matrimid® 5218 | Chloroform | Fillers dispersed in the solvent in an ultrasonic bath for 20 min and stirred overnight. Polymer added and stirred magnetically at room temperature for 24 h, followed by sonication. Solution cast on a Petri dish and left covered overnight followed by 24 h in a vacuum oven at 180 °C. | 0.17 ± 0.02 µm ZIF-8 4.3 ± 0.6 µm MSS-Z8 112 ± 10 µm ZIF-8 MMM125 µm MSS-Z8 MMM 12 wt% loading | 10/90 wt% water/ethanol mixtures at 42 °C ZIF-8 MMM Flux-260 g/m2 h SF-300 MSS-Z8 MM Flux-200 g/m2 h SF-137 | [7] |
| 2. | ZIF-71 Hydrophobic | Polyether-block-amide (PEBA) | n-butanol | ZIF-71 particles dispersed in n-butanol, stirred and sonicated alternatively for 1/2 h for three times each. “Primed” by adding a small amount of PEBA stirred at 80 °C for 4 h. Remaining polymer added and stirred for 4 h. Solution kept at 60 °C overnight. Membranes cast on PVDF substrate. After 2 days, dried in an oven at 70 °C for 24 h. | 1 µm particle size 10–20 µm thick membrane 20 wt% loading | Biobutanol recovery from acetone–butanol–ethanol (ABE) fermentation broth at 37 °C Flux-447.9 g/m2 h SF-18.4 | [14] |
| 3. | ZIF-8-NH2 | Poly(vinyl alcohol) (PVA) PVA/ZIF-8-NH2 | Water | PVA solution prepared at 90 °C by stirring and complete dissolution. ZIF-8 particle suspension in DI water prepared, shook for 1 h vigorously, and ultrasonicated for 1 h. Suspension added into PVA solution, stirred for 12 h vigorously ultrasonicated for 30 min. Overnight degassing. Solution cast on a polyethylene terephthalate (PET) plate and dried at room temperature under vacuum overnight. Peeled and dried at 50 °C for 12 h. | 200 nm-sized particles 15 µm thick membranes 7.5 wt% loading | Ethanol dehydration (85/15 wt% ethanol/water mixture) at 50 °C Flux-185 g/m2 h SF-119 85/15 wt% isopropanol/water mixture at 40 °C Flux-112 g/m2 h SF-1200 | [62] |
| 4. | ZIF-8 | Poly (vinyl alcohol)PVA/ZIF-8 | Water | Drying free process and water phase solution | 60 nm sized particles 20–50 µm thick membranes 0–39 wt% loading | Ethanol dehydration ethanol/water mixture (90:10 w/w) at 25 °C 39 wt% loading SF-4725 | [63] |
| 5. | ZIF-8 | Chitosan CS/ZIF-8 | Acetic acid | ZIF-8 heated at 120 °C under vacuum overnight, then dispersed in 2 wt% acetic acid aqueous solution. 10% of the desired amount of CS added to the ZIF-8/solvent suspension and stirred overnight. Remaining CS dissolved in the previously prepared solution to reach a CS concentration of 2 wt% and stirred for 24 h. The solution was degassed under vacuum (–0.04 MPa) at room temperature for 4 h and reacted with GA as a cross-linker. Defined volumes of the resulting solution cast on a glass plate and dried at room temperature for 48 h. | 60 nm sized particles 40–60 µm thick membrane 2.5, 5, 7.5, 10 wt% loading | Isopropanol dehydration 85 wt% IPA aqueous solution at 30 °C 5 wt% loading Flux-410 g/m2 h SF-723 | [64] |
| 6. | ZIF-7 | Chitosan ZIF-7/CS | Water, acetic acid | CS powders dissolved in DI with 2 wt% acetic acid. ZIF-7 particles (heated at 160 °C for 24 h) added, followed by vigorous stirring for 12 h and reaction with GA in an ice-water bath for 0.5 h. The solution was cast to form a thin film, dried in an oven at 45 °C overnight and then peeled. | 1–2 µm sized particles 18 µm thick membrane 2.5, 4, 5, 6 and 7.5 wt% loadings (optimum- 5 wt%) | 90 wt% ethanol aqueous solution at 25 °C 5 wt% loading: Flux-322 g/m2 h SF-2812 | [65] |
| 7. | ZIF-8 | Polyether block amide (PEBA-2533) | N,N-dimethyl acetamide | ZIF-8 particles (dried at 100 °C for 1 h in a vacuum oven) mixed with N,N-dimethyl acetamide, followed by stirring and sonication alternatively for 0.5 h for three times. PEBA-2533 added and stirred at 70 °C for 1.5 h and sonicated at 70 °C. Solution cast onto a glass plate at 70 °C, followed heating at 70 °C in an oven for 1 day, followed by a vacuum oven at 50 °C for about 24 h. | 100 nm sized particles 50 µm thick membrane | 0.8 wt% Phenol aqueous solution at 70 °C 10 wt% loading: Flux-1310 g/m2 h SF-53 | [66] |
| 8. | ZIF-90 | Polyvinylidene difluoride (PVDF) | DMF, acetone | PVDF dissolved in DMF. ZIF dissolved in acetone and ultrasonicated for 10 min. Both solutions mixed and ultrasonicated. Magnetic stirring on a hot plate for 50 °C. Solution cast on a glass substrate. Dried at 70 °C for 20 min. | 100 nm sized particles 40–60 µm thick membranes 5, 10, 20, 30 wt% loading | NA | [67] |
| 9. | ZIF-8 | Polybenzimidazole (PBI) PBI/ZIF-8 | 1-Methyl-2-pyrrolidinone (NMP) | PBI dissolved in NMP by stirring for 48 h at 120 °C, followed by cooling down to room temperature and filtration. ZIF-8 dispersed in NMP. Stirred and sonicated. Added into a PBI/NMP solution followed by stirring. Solution poured into a casting ring on a silica wafer and dried in a vacuum oven at 75 °C for 12 h. Membrane peeled and dried in a vacuum oven at 200 °C for 12 h. | 50 nm sized particles50 µm membrane thickness12.4, 27.4 33.7, 58.7 wt% loadings | Alcohol/water mixture of 85/15 wt/wt at 60 °C 33.7 wt%: IPAFlux-103 g/m2 h SF-1686 Ethanol Flux-81 g/m2 h SF-3417 n-butanol Flux-992 g/m2 h SF-10 58.7 wt%: IPA Flux-246 g/m2 h SF-310 n-butanol Flux-226 g/m2 h SF-698 | [68] |
| 10. | PEG-g-ZIF-8 Polyethylene glycol grafted ZIF-8 | Poly(vinyl alcohol) PEG-g-ZIF-8/PVA | Water | Solution casting and solvent evaporation method with GA as cross-linking agent. | 25 nm sized particles 40 µm thick membranes 5, 10, 15 wt% loading | 88:12 wt/wt Isopropanol/water solution at 25 °C 15 wt% loading Flux-91 g/m2 h SF-7326 | [69] |
| 11. | ZIF-8 | PVDF ZIF-8/gelatin/PVDF | Ethanol/water | ZIF-8 seeds/gelatin layer prepared by immersing ZHNs/gelatin/PVDF hollow fibre into Hmim ethanol/water solution at room temperature for 12 h followed by immersion into Zn(NO3)2·6H2O and Hmim solution at 30 °C for 6 h. | 2 µm thick membranes | Rhodamine B dye/water Rejection-90.5% Permeance-137 L/m2 h bar | [70] |
| 12. | GO@ZIF-67 | Polyacrylonitrile (PAN)GO@ZIF-67/PAN | DMF | Casting method. GO@ZIF-67 added to DMF and sonicated for 5 min. PAN powder added to the mixture followed by stirring for 12 h. Solution poured into a glass petri dish and dried at 80 °C for 4, cooled to room temperature and immersed in DI water and dried. | NA | Methylene blue/water Photocatalytic adsorption | [71] |
| 13. | ZIF-8 modified graphene oxide (ZGO) | Polyether block amide (PEBA) ZGO/PEBA | Methanol, n-butanol | ZGO in methanol and 2 h sonication and 4 h stirring. PEBA in n-butanol and 4 h mixing at 80 °C. ZGO laminates deposited on the surface of the ceramic substrates by vacuum-assisted assembly method in 3 min. Immersed into PEBA solution for 3 min. Stabilized with vacuum suction in air for 3 min. Dried for 5 h at different temperatures. | 1 µm thick membrane | 1% butanol from water at 75 °C Flux-606 g/m2 h SF-23.7 5% butanol from water at 55 °C Flux-1001 g/m2 h SF-29.3 | [72] |
| 14. | ZIF-90 | Poly(vinyl alcohol) (PVA) ZIF-90/PVA | Water | Viscosity-driven in situ self-assembly method. PVA dissolved in DI water at 90 °C for 1 h with stirring. ICA solution added at 60 °C with stirring for 30 min. Zn(NO3)2·6H2O solution added and stirred for 2 min. Solution cast onto the culture dish at room temperature. Peeled off. Thermal treatment at 90, 110, 130, and 150 °C in a vacuum oven for 2 h. | 350 nm sized particles 70–80 µm thick membrane | 90 wt% ethanol aqueous solution at 30 °C Flux-268 g/m2 h SF-1379 | [73] |
| 15. | ZIF-71 | PVDF ZIF-71/PVDF hollow fibre membrane | DMF | Dilute solution phase inversion process. PVDF powders and PEG-400 dissolved in DMF followed by adding a certain amount of ZIF-71. PVDF hollow fibre support membranes soaked in ethanol aqueous solution for 5 min and dried at room temperature and two ends sealed with silicone rubber. Membranes immersed in PVDF dilute solution for 10 s, and immediately immersed in pure water for 24 h. | 0.7–1.2 µm sized particles 1 µm thick selective layer 0–2 wt% loading | VMD desalination Flux-27.1 kg/m2 h | [74] |
| 16. | ZIF-8@RMs ZIF-8 on resin microspeheres | PolyphenylsulfonePPSU/ZIF-8@RMs | NMP | Phase inversion. PPSU dissolved in NMP and ZIF-8@RMs added into the solution. Ultrasonication for 30 min, stirring for 12 h at 25 °C and left overnight. Suspension was cast onto a clean rectangular steel plate at room temperature, immersed in a DI water coagulation bath for 15 min. Placed in freshwater for 24. Dried in air at room temperature overnight. | 10 µm sized particles 100 µm thick membranes 5 wt% loading | Dye rejection from methanol solutions | [75] |
| 17. | ZIF-L | Polyethersulfone (PES)PES/ZIF-L | NMP | Non-solvent induced phase separation method at room temperature. PVP powder and methanol-wetted ZIF-L nanoflakes mixed into NMP, ultrasonication for 10 min, followed by stirring for 30 min. PES added. Overnight stirring and degassing for 8 h. Membrane cast on a glass plate at room temperature and immersed in DI water for 24 h. | 0.25, 0.5, 1 wt% loadings (0.5% optimum) Leaf-like nanoflakes with 150 nm thickness | Improvement of water flux | [76] |
| 18. | ZIF-8 | Polyethyleneglycol (PEG) | Water | ZIF-8 particles dispersion and PEG aqueous solution mixed and stirred. Maleic anhydride (crosslinking agent) and trimethylamine (catalyst) added with magnetic stirring. Solution cast on a PVDF supporting membrane and kept at room temperature for 12 h, followed by cross-linking for 5 h at 80 °C. | 0, 2, 4, 6 wt% loading (optimum- 4 wt%) | Desulphurisation Thiophene/n-heptane mixture Flux-1960 g/m2 h Enrichment factor-8.93 | [77] |
| 19. | Carbonized ZIF-8 (CZIF-8) | Polyimide (PI) CZIF-8/PI | Water | Non-solvent induced phase separation (NIPS) method was used. PAA solution with CZIF-8 stirred at room temperature for 5 h, followed by sonication for 30 min and degassing in vacuum for 30 min. Solution cast on the PET non-woven fabric, immersed in DI water coagulation bath for 30 min, dried and thermally treated under nitrogen. | 0–20 wt% loading (10 wt% optimum) | Nanofiltration performance; rejection of dyes (rose bengal, methyl blue, congo red, chrome black T) from aqueous and alcoholic (ethanol, isopropanol) solutions Approx-95% rejection of congo red from alcoholic solutions | [78] |
| 20. | β-cyclodextrin-enhanced ZIF-8 (β-CD@ZIF-8) | poly(m-phenylene isophthalamide) (PMIA) | NA | The PMIA support layer formed onto the PI nanofiber (formed by electrospinning) by NIPS method and spin coating of PMIA with a spinner and IP between mphenylenediamine (MPD) and trimesoyl chloride (TMC) with the improvement of β-CD@ZIF-8. | 87.1 ± 10.7 nm sized particles 102.1 ± 4.0 nm thick selective layer0.05 wt% loading | Organic solvent nanofiltration (dye/solvent mixtures) | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, P.; Sundarrajan, S.; Ramakrishna, S. A Review on Mixed Matrix Membranes for Solvent Dehydration and Recovery Process. Membranes 2021, 11, 441. https://doi.org/10.3390/membranes11060441
Goyal P, Sundarrajan S, Ramakrishna S. A Review on Mixed Matrix Membranes for Solvent Dehydration and Recovery Process. Membranes. 2021; 11(6):441. https://doi.org/10.3390/membranes11060441
Chicago/Turabian StyleGoyal, Priyanka, Subramanian Sundarrajan, and Seeram Ramakrishna. 2021. "A Review on Mixed Matrix Membranes for Solvent Dehydration and Recovery Process" Membranes 11, no. 6: 441. https://doi.org/10.3390/membranes11060441
APA StyleGoyal, P., Sundarrajan, S., & Ramakrishna, S. (2021). A Review on Mixed Matrix Membranes for Solvent Dehydration and Recovery Process. Membranes, 11(6), 441. https://doi.org/10.3390/membranes11060441