MnO2@Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for the Simultaneous Determination of Trace Cd(II), Zn(II) and Cu(II) in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Preparation of Reduced Graphene Oxide (RGO)
2.4. Preparation of Manganese Oxide Nanoparticles (MnO2)
2.5. Preparation of the MnO2@RGO/GCE Modified Electrode
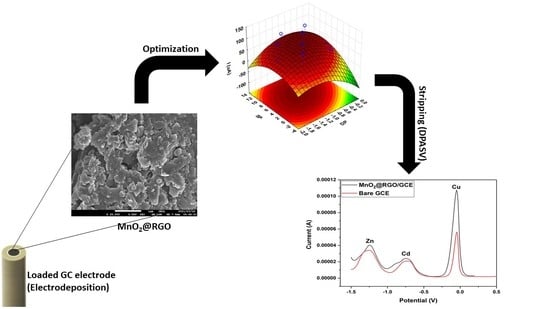
3. Results
3.1. X-ray Diffraction (XRD)
3.2. High Resolution-Scanning Electron Microscopy (HR-SEM)
3.3. Transmission Electron Microscopy (TEM)
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Nitrogen Absorption-Desorption Studies
3.6. Electrochemical Performance of the Sensing Materials
3.7. Optimization of the DPASV Experimental Parameters
3.8. Analytical Figures of Merit
3.9. Surface Water (SW) Sample Analysis
3.10. Stability of the Electrochemical Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors 2021, 21, 131. [Google Scholar] [CrossRef]
- Swierczewska, M.; Liu, G.; Lee, S.; Chen, X. High-sensitivity nanosensors for biomarker detection. Chem. Soc. Rev. 2012, 41, 2641–2655. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, H.; Li, M.; Wang, G.; Long, Y.; Li, P.; Li, C.; Yang, B. Polydopamine/graphene/MnO2 composite-based electrochemical sensor for in situ determination of free tryptophan in plants. Anal. Chim. Acta 2021, 1145, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clin. Toxicol. 2011, 3, 495. [Google Scholar] [CrossRef] [Green Version]
- Obaroh, I.O.; Abubakar, U.; Haruna, M.A.; Elinge, M.C. Evaluation of some heavy metals concentration in River Argungu. J. Fish. Aquat. Sci. 2015, 10, 581. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, H.; Chai, L.; Li, M. Effects of single-and multi-organic acid ligands on adsorption of copper by Fe3O4/graphene oxide-supported DCTA. J. Colloid Interface Sci. 2016, 478, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135. [Google Scholar]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Thanh, N.M.; Van Hop, N.; Luyen, N.D.; Phong, N.H.; Tam Toan, T.T. Simultaneous determination of Zn (II), Cd (II), Pb (II), and Cu (II) using differential pulse anodic stripping voltammetry at a bismuth film-modified electrode. Adv. Mater. Sci. Eng. 2019, 2019, 1826148. [Google Scholar] [CrossRef] [Green Version]
- Alam, R.; Ahmed, Z.; Howladar, M.F. Evaluation of heavy metal contamination in water, soil and plant around the open landfill site Mogla Bazar in Sylhet, Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100311. [Google Scholar] [CrossRef]
- Rofouei, M.K.; Jamshidi, S.; Seidi, S.; Saleh, A. A bucky gel consisting of Fe3O4 nanoparticles, graphene oxide and ionic liquid as an efficient sorbent for extraction of heavy metal ions from water prior to their determination by ICP-OES. Microchim. Acta 2017, 184, 3425–3432. [Google Scholar] [CrossRef]
- Gugushe, A.S.; Mpupa, A.; Munonde, T.S.; Nyaba, L.; Nomngongo, P.N. Adsorptive Removal of Cd, Cu, Ni and Mn from Environmental Samples Using Fe3O4-Zro2@ APS Nanocomposite: Kinetic and Equilibrium Isotherm Studies. Molecules 2021, 26, 3209. [Google Scholar] [CrossRef]
- Alexander, D.; Ellerby, R.; Hernandez, A.; Wu, F.; Amarasiriwardena, D. Investigation of simultaneous adsorption properties of Cd, Cu, Pb and Zn by pristine rice husks using ICP-AES and LA-ICP-MS analysis. Microchem. J. 2017, 135, 129–139. [Google Scholar] [CrossRef]
- Nomngongo, P.N.; Ngila, J.C. Multivariate optimization of dual-bed solid phase extraction for preconcentration of Ag, Al, As and Cr in gasoline prior to inductively coupled plasma optical emission spectrometric determination. Fuel 2015, 139, 285–291. [Google Scholar] [CrossRef]
- Akanji, S.P.; Ama, O.M.; Ray, S.S.; Osifo, P.O. Metal Oxide Nanomaterials for Electrochemical Detection of Heavy Metals in Water. In Nanostructured Metal-Oxide Electrode Materials for Water Purification; Springer: Berlin/Heidelberg, Germany, 2020; pp. 113–126. [Google Scholar]
- Ramachandran, R.; Chen, T.-W.; Chen, S.-M.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Pujol, L.; Evrard, D.; Groenen-Serrano, K.; Freyssinier, M.; Ruffien-Cizsak, A.; Gros, P. Electrochemical sensors and devices for heavy metals assay in water: The French groups’ contribution. Front. Chem. 2014, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Sawan, S.; Maalouf, R.; Errachid, A.; Jaffrezic-Renault, N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC Trends Anal. Chem. 2020, 131, 116014. [Google Scholar] [CrossRef]
- Tan, Z.; Wu, W.; Feng, C.; Wu, H.; Zhang, Z. Simultaneous determination of heavy metals by an electrochemical method based on a nanocomposite consisting of fluorinated graphene and gold nanocage. Microchim. Acta 2020, 187, 414. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Penicillamine-modified sensor for the voltammetric determination of Cd (II) and Pb (II) ions in natural samples. Talanta 2015, 144, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Zhou, T.; Song, D.; Zhang, L.; Hu, X. Stripping voltammetric detection of mercury (II) based on a bimetallic Au-Pt inorganic-organic hybrid nanocomposite modified glassy carbon electrode. Anal. Chem. 2010, 82, 567–573. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Anodic stripping voltammetric determination of traces of Pb (II) and Cd (II) using a glassy carbon electrode modified with bismuth nanoparticles. Microchim. Acta 2014, 181, 1199–1206. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Priya, T.; Dhanalakshmi, N.; Thennarasu, S.; Karthikeyan, V.; Thinakaran, N. Ultra sensitive electrochemical detection of Cd2+ and Pb2+ using penetrable nature of graphene/gold nanoparticles/modified L-cysteine nanocomposite. Chem. Phys. Lett. 2019, 731, 136621. [Google Scholar] [CrossRef]
- Guo, Z.; Luo, X.; Li, Y.; Zhao, Q.-N.; Li, M.; Zhao, Y.; Sun, T.; Ma, C. Simultaneous determination of trace Cd (II), Pb (II) and Cu (II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J. Colloid Interface Sci. 2017, 490, 11–22. [Google Scholar] [CrossRef]
- Baghayeri, M.; Ghanei-Motlagh, M.; Tayebee, R.; Fayazi, M.; Narenji, F. Application of graphene/zinc-based metal-organic framework nanocomposite for electrochemical sensing of As (III) in water resources. Anal. Chim. Acta 2020, 1099, 60–67. [Google Scholar] [CrossRef]
- Lin, H.; Li, M.; Mihailovič, D. Simultaneous determination of copper, lead, and cadmium ions at a Mo6S9-xIx nanowires modified glassy carbon electrode using differential pulse anodic stripping voltammetry. Electrochim. Acta 2015, 154, 184–189. [Google Scholar] [CrossRef]
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J. Hazard. Mater. 2017, 324, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Mansha, M.; Ullah, N. Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction. TrAC Trends Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Brown, B.; Cordova, I.A.; Parker, C.B.; Stoner, B.R.; Glass, J.T. Optimization of active manganese oxide electrodeposits using graphenated carbon nanotube electrodes for supercapacitors. Chem. Mater. 2015, 27, 2430–2438. [Google Scholar] [CrossRef]
- Wang, M.-F.; Li, W.; Hu, P.-J.; He, S.-S.; Yang, H.-M.; Li, X.-Z. A Facile Hydrazine Amperometric Sensor Based on a Glassy Carbon Electrode Modified with Au Nanoparticles-MnO2 Composites. Int. J. Electrochem. Sci. 2016, 11, 1928–1937. [Google Scholar]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Le, T.-H.; Ngo, T.H.A.; Doan, V.T.; Nguyen, L.M.T.; Le, M.C. Preparation of manganese dioxide nanoparticles on laterite for methylene blue degradation. J. Chem. 2019, 2019, 1602752. [Google Scholar] [CrossRef] [Green Version]
- Shinde, P.A.; Lokhande, V.C.; Patil, A.M.; Yadav, A.A.; Lokhande, C.D. Hydrothermal synthesis of manganese oxide thin films using different oxidizing agents for supercapacitor application. Int. J. Eng. Res. Technol. 2017, 10, 532–537. [Google Scholar]
- Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Li, G.; Liu, J. Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Sannasi, V.; Subbian, K. Influence of Moringa oleifera gum on two polymorphs synthesis of MnO 2 and evaluation of the pseudo-capacitance activity. J. Mater. Sci. Mater. Electron. 2020, 31, 17120–17132. [Google Scholar] [CrossRef]
- Furlan, A.; Lu, J.; Hultman, L.; Jansson, U.; Magnuson, M. Crystallization characteristics and chemical bonding properties of nickel carbide thin film nanocomposites. J. Phys. Condens. Matter 2014, 26, 415501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Le Flem, M.; Béchade, J.-L.; Onimus, F.; Cozzika, T.; Monnet, I. XRD investigation of ion irradiated Ti3Si0. 90Al0. 10C2. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 506–512. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, X.; Cai, J.; Liu, W.; Lin, S. A novel MnO 2/rGO composite prepared by electrodeposition as a non-noble metal electrocatalyst for ORR. J. Appl. Electrochem. 2019, 49, 767–777. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, Y.; Liu, J.; Wang, J.; Zhang, B.; Xiang, X. Ultrafine MnO2 nanoparticles decorated on graphene oxide as a highly efficient and recyclable catalyst for aerobic oxidation of benzyl alcohol. J. Colloid Interface Sci. 2016, 483, 26–33. [Google Scholar] [CrossRef]
- Mylarappa, M.; Lakshmi, V.V.; Mahesh, K.R.V.; Nagaswarupa, H.P.; Raghavendra, N. A facile hydrothermal recovery of nano sealed MnO2 particle from waste batteries: An advanced material for electrochemical and environmental applications. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 149, p. 12178. [Google Scholar]
- Hossain, M.N.; Wen, J.; Chen, A. Unique copper and reduced graphene oxide nanocomposite toward the efficient electrochemical reduction of carbon dioxide. Sci. Rep. 2017, 7, 3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mpupa, A.; Nqombolo, A.; Mizaikoff, B.; Nomngongo, P.N. Enhanced Adsorptive Removal of β-Estradiol from Aqueous and Wastewater Samples by Magnetic Nano-Akaganeite: Adsorption Isotherms, Kinetics, and Mechanism. Processes 2020, 8, 1197. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Simultaneous detection of Cd (II) and Pb (II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Rajabi, M.; Mohammadi, B.; Asghari, A.; Barfi, B.; Behzad, M. Nano-alumina coated with SDS and modified with salicylaldehyde-5-sulfonate for extraction of heavy metals and their determination by anodic stripping voltammetry. J. Ind. Eng. Chem. 2014, 20, 3737–3743. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Dai, X.; Zhang, Z.; Ding, F.; Li, S. An ultrasensitive electrochemical platform based on imprinted chitosan/gold nanoparticles/graphene nanocomposite for sensing cadmium (II) ions. Microchem. J. 2020, 155, 104710. [Google Scholar] [CrossRef]
- Xie, R.; Zhou, L.; Lan, C.; Fan, F.; Xie, R.; Tan, H.; Xie, T.; Zhao, L. Nanostructured carbon black for simultaneous electrochemical determination of trace lead and cadmium by differential pulse stripping voltammetry. R. Soc. Open Sci. 2018, 5, 180282. [Google Scholar] [CrossRef] [Green Version]










| Surface Properties | RGO Nanosheets | MnO2 Nanoparticles | MnO2@RGO Nanocomposite |
|---|---|---|---|
| BET surface area (m2/g) | 54.1 | 77.5 | 148.7 |
| Average pore size (nm) | 14.1 | 18.8 | 26.6 |
| Total pore volume (cm3/g) | 0.19 | 0.33 | 0.77 |
| Analytical Parameters | Cd | Cu | Zn |
|---|---|---|---|
| DLR (μg L−1) | LOQ-700 | LOQ-600 | LOQ-600 |
| R2 | 0.9967 | 0.9990 | 0.9987 |
| RLE | |||
| LOD (ng L−1) | 15.1 | 9.3 | 13.7 |
| LOQ (ng L−1) | 50.3 | 31.0 | 44.3 |
| Intraday (%RSD) | 2.1 | 1.5 | 2.5 |
| Interday (%RSD) | 3.9 | 4.0 | 4.5 |
| Electrode/Method | Analytes | LOD (μg L−1) | LOQ(μg L−1) | DLR (μg L−1) | RSD % | Ref |
|---|---|---|---|---|---|---|
| Mo6SxI9-xNWs/GCE/DPASV | Cu and Cd | 0.015 | 0.50–240 | 3.3–5.1 | [27] | |
| BiFE/GCE | Cu | 0.028 | 5–110 | - | [9] | |
| Nafion/Bi/NMC/GCE/DPASV | Cu, Cd, Zn | 0.93–1.07 | 2–100 | 7.5 | [47] | |
| RGO-CS/PLL/GCE | Cd and Cu | 0.01–0.02 | 0.05–10. | 4.4 | [25] | |
| Nano-Al2O/GCE/DPV | Zn | 0.026 | 0.08–150 | 3.39 | [48] | |
| CS/AuNPs/GR/GCE | Cd | 0.002 | 0.1–0.9 | 2.4 | [49] | |
| CB–Nafion–GCE | Cd | 0.9 | 0.6–112 | 3.13 | [50] | |
| MnO2@RGO/GCE/DPV | Cd, Zn and Cu | 0.002–0.015 | 0.021–0.050 | 0.05–700 | 1.5–4.5 | This study |
| Samples | Added | Cd | Cu | Zn | |||
|---|---|---|---|---|---|---|---|
| Found | %R | Found | %R | Found | %R | ||
| SW1 | 0 | 1.32 ± 0.05 | - | 56.7 ± 0.9 | - | 126.5 ± 1.8 | - |
| 2.0 | 3.27 ± 0.02 | 97.5 | 58.7 ± 1.0 | 99.0 | 128.5 ± 1.3 | 98.5 | |
| 5.0 | 6.18 ± 0.12 | 97.2 | 61.6 ± 0.8 | 99.0 | 131.4 ± 1.6 | 98.8 | |
| 10.0 | 11.2 ± 0.17 | 98.5 | 66.6 ± 0.9 | 99.0 | ±1.5 | 99.8 | |
| SW2 | 0 | 2.45 ± 0.02 | - | 102.4 ± 1.5 | - | 337.1 ± 3.1 | - |
| 2.0 | 4.43 ± 0.07 | 99.0 | 104.4 ± 1.4 | 99.0 | 339.0 ± 2.3 | 97.0 | |
| 5.0 | 7.37 ± 0.06 | 98.2 | 107.3 ± 1.6 | 99.2 | 342.1 ± 3.1 | 99.2 | |
| 10.0 | 12.7 ± 0.80 | 99.1 | 112.4 ± 1.2 | 99.6 | 347.0 ± 2.5 | 99.3 | |
| SW3 | 0 | 1.66 ± 0.06 | - | 125.9 ± 1.3 | - | 231.0 ± 2.4 | - |
| 2.0 | 3.63 ± 0.05 | 98.5 | 127.9 ± 1.1 | 98.5 | 232.0 ± 2.5 | 99.0 | |
| 5.0 | 6.61 ± 0.16 | 99.0 | 130.9 ± 1.3 | 99.6 | 235.0 ± 2.6 | 99.4 | |
| 10.0 | 11.6 ± 0.23 | 99.4 | 135.8 ± 1.5 | 99.0 | 240.9 ± 2.7 | 99.4 | |
| Samples | Concentration (µg L−1) | ICP-OES (Concentration (µg L−1) | ||||
|---|---|---|---|---|---|---|
| Cd | Cu | Zn | Cd | Cu | Zn | |
| SW4 | <LOD | 130.4 ± 1.2 | 231.6 ± 3.0 | <LOD | 129.5 ± 2.3 | 231.6 ± 3.0 |
| SW5 | 0.98 ± 0.01 | 75.4 ± 0.9 | 144.3 ± 1.8 | 1.00 ± 0.01 | 74.6 ± 2.2 | 143.5 ± 2.3 |
| SW6 | 1.23 ± 0.06 | 29.6 ± 0.5 | 89.6 ± 0.8 | 1.21 ± 0.07 | 30.1 ± 1.3 | 90.3 ± 1.2 |
| SW7 | 1.03 ± 0.02 | 238.1 ± 2.6 | 92.3 ± 1.0 | 1.00 ± 0.12 | 238.5 ± 1.6 | 91.8 ± 1.8 |
| SW8 | 2.31 ± 0.05 | 77.5 ± 1.2 | 136.7 ± 2.1 | 2.34 ± 0.09 | 78.1 ± 1.8 | 137.0 ± 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnyipika, S.H.; Munonde, T.S.; Nomngongo, P.N. MnO2@Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for the Simultaneous Determination of Trace Cd(II), Zn(II) and Cu(II) in Water Samples. Membranes 2021, 11, 517. https://doi.org/10.3390/membranes11070517
Mnyipika SH, Munonde TS, Nomngongo PN. MnO2@Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for the Simultaneous Determination of Trace Cd(II), Zn(II) and Cu(II) in Water Samples. Membranes. 2021; 11(7):517. https://doi.org/10.3390/membranes11070517
Chicago/Turabian StyleMnyipika, Siyamthanda Hope, Tshimangadzo Saddam Munonde, and Philiswa Nosizo Nomngongo. 2021. "MnO2@Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for the Simultaneous Determination of Trace Cd(II), Zn(II) and Cu(II) in Water Samples" Membranes 11, no. 7: 517. https://doi.org/10.3390/membranes11070517
APA StyleMnyipika, S. H., Munonde, T. S., & Nomngongo, P. N. (2021). MnO2@Reduced Graphene Oxide Nanocomposite-Based Electrochemical Sensor for the Simultaneous Determination of Trace Cd(II), Zn(II) and Cu(II) in Water Samples. Membranes, 11(7), 517. https://doi.org/10.3390/membranes11070517