Effect of Organic Substances on Nutrients Recovery by Struvite Electrochemical Precipitation from Synthetic Anaerobically Treated Swine Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Characterisation
2.2. Experiment Setup
2.3. Batch Experiments
2.4. Analytical Methods
3. Results and Discussion
3.1. Effect of the Initial pH and Current Density on Struvite Electrochemical Precipitation Performance
3.1.1. Nutrients Removal from Synthetic Wastewater Containing Only Orthophosphate Phosphorus and Ammonium Nitrogen
3.1.2. Precipitate Analysis from Synthetic Wastewater Containing Only Orthophosphate Phosphorus and Ammonium Nitrogen
3.2. Effect of Organic Substances on Struvite Electrochemical Precipitation Performance
3.2.1. Nutrients Removal from Synthetic Wastewater Containing Orthophosphate Phosphorus, Ammonium Nitrogen and Organics with Major Ions
3.2.2. Precipitate Analysis from Synthetic Wastewater Containing Orthophosphate Phosphorus, Ammonium Nitrogen and Organics with Major Ions
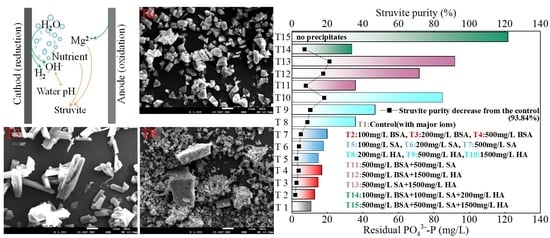
3.3. Preliminary Tests to Mitigate the Adverse Effect of Organic Substances on Struvite Electrochemical Precipitation Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shim, S.; Reza, A.; Kim, S.; Won, S.; Ra, C. Nutrient recovery from swine wastewater at full-scale: An integrated technical, economic and environmental feasibility assessment. Chemosphere 2021, 277, 130309. [Google Scholar] [CrossRef]
- Cheng, D.K.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 2018, 621, 1664–1682. [Google Scholar] [CrossRef]
- Domingues, E.; Fernandes, E.; Gomes, J.; Martins, R.C. Swine wastewater treatment by Fenton’s process and integrated methodologies involving coagulation and biofiltration. J. Clean. Prod. 2021, 293, 126105. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, P.; Chen, Z. Anaerobic digestion and post-treatment of swine wastewater using IC-SBR process with bypass of raw wastewater. Process. Biochem. 2006, 41, 965–969. [Google Scholar] [CrossRef]
- Bernet, N.; Delgenes, N.; Akunna, J.C.; Delgenes, J.P.; Moletta, R. Combined anaerobic-aerobic SBR for the treatment of piggery wastewater. Water Res. 2000, 34, 611–619. [Google Scholar] [CrossRef]
- Deng, K.; Tang, L.; Li, J.; Meng, J.; Li, J. Practicing anammox in a novel hybrid anaerobic-aerobic baffled reactor for treating high-strength ammonium piggery wastewater with low COD/TN ratio. Bioresour. Technol. 2019, 294, 122193. [Google Scholar] [CrossRef]
- Padmasiri, S.I.; Zhang, J.; Fitch, M.; Norddahl, B.; Morgenroth, E.; Raskin, L. Methanogenic population dynamics and performance of an anaerobic membrane bioreactor (AnMBR) treating swine manure under high shear conditions. Water Res. 2007, 41, 134–144. [Google Scholar] [CrossRef]
- Song, X.; Luo, W.; McDonald, J.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. An anaerobic membrane bioreactor-membrane distillation hybrid system for energy recovery and water reuse: Removal performance of organic carbon, nutrients, and trace organic contaminants. Sci. Total Environ. 2018, 628–629, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Muhmood, A.; Wang, X.; Dong, R.; Wu, S. New insights into interactions of organic substances in poultry slurry with struvite formation: An overestimated concern? Sci. Total Environ. 2021, 751, 141789. [Google Scholar] [CrossRef]
- Pastor, L.; Mangin, D.; Barat, R.; Seco, A. A pilot-scale study of struvite precipitation in a stirred tank reactor: Conditions influencing the process. Bioresour. Technol. 2008, 99, 6285–6291. [Google Scholar] [CrossRef]
- Tao, W.; Fattah, K.P.; Huchzermeier, M.P. Struvite recovery from anaerobically digested dairy manure: A review of application potential and hindrances. J. Environ. Manag. 2016, 169, 46–57. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.M.; Boiarkina, I.; Yu, W.; Huang, Y.F.; Wang, G.Q.; Young, B.R. Phosphorus recovery through struvite crystallisation: Recent developments in the understanding of operational factors. J. Environ. Manag. 2019, 248, 109254. [Google Scholar] [CrossRef]
- Kruk, D.J.; Elektorowicz, M.; Oleszkiewicz, J.A. Struvite precipitation and phosphorus removal using magnesium sacrificial anode. Chemosphere 2014, 101, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, Y.; Hu, Q.; Yu, X.; Qian, F. Effects of three kinds of organic acids on phosphorus recovery by magnesium ammonium phosphate (MAP) crystallization from synthetic swine wastewater. Chemosphere 2014, 101, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Hong, T.; Cui, K.; Chen, T.; Zhou, Y.; Zhao, Y.; Yin, Y.; Wang, J.; Zhang, Q. Probing the effect of humic acid on the nucleation and growth kinetics of struvite by constant composition technique. Chem. Eng. J. 2019, 378, 122130. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, D.; Ren, W.; Zhao, Y.; Jiang, L.-M.; Wang, L. Effect of humic substances on phosphorus removal by struvite precipitation. Chemosphere 2015, 141, 94–99. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Wang, S.; Jiang, Y.; Xiao, D.; Ding, L.; Gao, F. Nutrients removal from swine wastewater by struvite precipitation recycling technology with the use of Mg3(PO4)2 as active component. Ecol. Eng. 2016, 92, 111–118. [Google Scholar] [CrossRef]
- Lin, X.; Han, Z.; Yu, H.; Ye, Z.; Zhu, S.; Zhu, J. Struvite precipitation from biogas digestion slurry using a two-chamber electrolysis cell with a magnesium anode. J. Clean. Prod. 2018, 174, 1598–1607. [Google Scholar] [CrossRef]
- Chen, R.-F.; Wu, L.; Zhong, H.-T.; Liu, C.-X.; Qiao, W.; Wei, C.-H. Evaluation of electrocoagulation process for high-strength swine wastewater pretreatment. Sep. Purif. Technol. 2021, 272, 118900. [Google Scholar] [CrossRef]
- Foletto, E.L.; dos Santos, W.R.B.; Jahn, S.L.; Bassaco, M.M.; Mazutti, M.A.; Cancelier, A.; Gundel, A. Organic pollutants removal and recovery from animal wastewater by mesoporous struvite precipitation. Desalin. Water Treat. 2013, 51, 2776–2780. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association, Water Environment Federation, American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Hao, X.D.; Wang, C.C.; Lan, L.; van Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Zhang, Z.; Liu, J.; Xiao, J.; Gao, F. Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. J. Clean. Prod. 2016, 127, 302–310. [Google Scholar] [CrossRef]
- Oumar, D.; Patrick, D.; Gerardo, B.; Rino, D.; Ihsen, B.S. Coupling biofiltration process and electrocoagulation using magnesium-based anode for the treatment of landfill leachate. J. Environ. Manag. 2016, 181, 477–483. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Gunay, A.; Karadag, D.; Tosun, I.; Ozturk, M. Use of magnesit as a magnesium source for ammonium removal from leachate. J. Hazard. Mater. 2008, 156, 619–623. [Google Scholar] [CrossRef]
- Kemacheevakul, P.; Polprasert, C.; Shimizu, Y. Phosphorus recovery from human urine and anaerobically treated wastewater through pH adjustment and chemical precipitation. Environ. Technol. 2011, 32, 693–698. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martinez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, Y.; Yuan, P.; Peng, J.; Fan, M. Modeling the crystallization of magnesium ammonium phosphate for phosphorus recovery. Chemosphere 2006, 65, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; He, X.; Wang, Z.; Chun, Z. Factors influencing the removal of phosphorus and the purity of recycling struvite in wastewater by the electrochemical sacrificial magnesium anode method. Sci. Adv. Mater. 2019, 11, 128–134. [Google Scholar] [CrossRef]
- Wang, C.C.; Hao, X.D.; Guo, G.S.; van Loosdrecht, M.C.M. Formation of pure struvite at neutral pH by electrochemical deposition. Chem. Eng. J. 2010, 159, 280–283. [Google Scholar] [CrossRef]
- Baigorri, R.; Fuentes, M.; Gonzalez-Gaitano, G.; Garcia-Mina, J.M.; Almendros, G.; Gonzalez-Vila, F.J. Complementary multianalytical approach to study the distinctive structural features of the main humic fractions in solution: Gray humic acid, brown humic acid, and fulvic acid. J. Agric. Food Chem. 2009, 57, 3266–3272. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.-F.; Liu, T.; Rong, H.-W.; Zhong, H.-T.; Wei, C.-H. Effect of Organic Substances on Nutrients Recovery by Struvite Electrochemical Precipitation from Synthetic Anaerobically Treated Swine Wastewater. Membranes 2021, 11, 594. https://doi.org/10.3390/membranes11080594
Chen R-F, Liu T, Rong H-W, Zhong H-T, Wei C-H. Effect of Organic Substances on Nutrients Recovery by Struvite Electrochemical Precipitation from Synthetic Anaerobically Treated Swine Wastewater. Membranes. 2021; 11(8):594. https://doi.org/10.3390/membranes11080594
Chicago/Turabian StyleChen, Run-Feng, Tao Liu, Hong-Wei Rong, Hai-Tao Zhong, and Chun-Hai Wei. 2021. "Effect of Organic Substances on Nutrients Recovery by Struvite Electrochemical Precipitation from Synthetic Anaerobically Treated Swine Wastewater" Membranes 11, no. 8: 594. https://doi.org/10.3390/membranes11080594
APA StyleChen, R. -F., Liu, T., Rong, H. -W., Zhong, H. -T., & Wei, C. -H. (2021). Effect of Organic Substances on Nutrients Recovery by Struvite Electrochemical Precipitation from Synthetic Anaerobically Treated Swine Wastewater. Membranes, 11(8), 594. https://doi.org/10.3390/membranes11080594