Comparative Study of the Structural and Functional Properties of Membrane-Isolated and Isoelectric pH Precipitated Green Lentil Seed Protein Isolates
Abstract
:1. Introduction
2. Materials and Methods
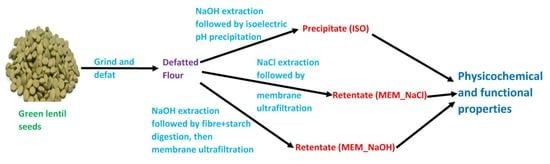
2.1. Raw Materials and Sample Preparation
2.1.1. Isoelectric Precipitation of Lentil Seed Proteins
2.1.2. Preparation of Lentil Seed Protein Isolates Using the Membrane Isolation Method
2.2. Physicochemical and Functional Properties of Isolated Lentil Proteins
2.2.1. Proximate Composition Analysis
2.2.2. Amino Acid Composition
2.2.3. Gel Electrophoresis
2.2.4. Intrinsic Fluorescence
2.2.5. Surface Hydrophobicity
2.2.6. Protein Secondary and Tertiary Structure Measurements by Circular Dichroism (CD)
2.2.7. Differential Scanning Calorimetry
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. Protein Solubility
2.2.10. Heat Coagulability (HC)
2.2.11. Emulsion Formation and Stability
2.2.12. Foam Formation and Stability
2.2.13. Water and Oil Holding Capacity
2.2.14. Least Gelation Concentration (LGC)
2.2.15. In Vitro Protein Digestibility
2.2.16. Statistical Analysis
3. Results
3.1. Proximate Composition
3.2. Amino Acid Composition
3.3. Molecular Weight (MW) Analysis
3.4. Intrinsic Fluorescence
3.5. Circular Dichroism (CD)
3.6. Scanning Electron Microscopy (SEM)
3.7. Differential Scanning Calorimetry (DSC)
3.8. Protein Digestibiltiy and Yield
3.9. Surface Hydrophobicity (So)
3.10. Least Gelation Concentration (LGC), Water (WHC), and Oil Holding Capacity (OHC)
3.11. Solubility as a Function of pH
3.12. Heat Coagulability (HC)
3.13. Emulsion Formation and Stability
3.14. Foaming Capacity (FC) and Foam Stability (FS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, M.; Timilsena, Y.; Adhikari, B. Global Production, Processing and Utilization of Lentil: A Review. J. Integr. Agric. 2017, 16, 2898–2913. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse Proteins: Secondary Structure, Functionality and Applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural Aspects of Legume Proteins and Nutraceutical Properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Duranti, M. Grain Legume Proteins and Nutraceutical Properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Physicochemical and Functional Properties of Kidney Bean Albumin and Globulin Protein Fractions. Food Res. Int. 2012, 48, 299–306. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 5 January 2021).
- Thavarajah, D.; Thavarajah, P.; Sarker, A.; Vandenberg, A. Lentils (Lens culinaris Medikus Subspecies Culinaris): A Whole Food for Increased Iron and Zinc Intake. J. Agric. Food Chem. 2009, 57, 5413–5419. [Google Scholar] [CrossRef]
- WHO; FAO. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007; Volume 935, ISBN 92-4-120935-6. [Google Scholar]
- Kinsella, J.E.; Melachouris, N. Functional Properties of Proteins in Foods: A Survey. Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Johnston, S.P.; Nickerson, M.T.; Low, N.H. The Physicochemical Properties of Legume Protein Isolates and Their Ability to Stabilize Oil-in-Water Emulsions with and without Genipin. J. Food Sci. Technol. 2015, 52, 4135–4145. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed Protein of Lentils: Current Status, Progress, and Food Applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Boye, J.I.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Malomo, S.A.; He, R.; Aluko, R.E. Structural and Functional Properties of Hemp Seed Protein Products. J. Food Sci. 2014, 79, C1512–C1521. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Miravalles, L.; Jeske, S.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; Wriessnegger, C.L.; O’Mahony, J.A.; Zannini, E.; Arendt, E.K. Membrane Filtration and Isoelectric Precipitation Technological Approaches for the Preparation of Novel, Functional and Sustainable Protein Isolate from Lentils. Eur. Food Res. Technol. 2019, 245, 1855–1869. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Effects of NaCl and pH on the Structural Conformations of Kidney Bean Vicilin. Food Chem. 2013, 139, 624–630. [Google Scholar] [CrossRef]
- Horwitz, W.; Chemists, A. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz; AOAC International: Gaithersburg, MD, USA, 2010; ISBN 978-0-935584-67-7. [Google Scholar]
- Mehlenbacher, V.C.; Sallee, E.M.; Hopper, T.H.; Link, W.E.; Walker, R.O.; Firestone, D. Official Methods and Recommended Practices of the AOCS, 3rd Printing, 6th ed.; AOCS Press: Urbana, IL, USA, 2010; ISBN 978-1-893997-74-5. [Google Scholar]
- Bidlingmeyer, B.A.; Cohen, S.A.; Tarvin, T.L. Rapid Analysis of Amino Acids Using Pre-Column Derivatization. J. Chromatogr. B Biomed. Sci. Appl. 1984, 336, 93–104. [Google Scholar] [CrossRef]
- Gehrke, C.W.; Wall Sr, L.L.; Absheer, J.S.; Kaiser, F.E.; Zumwalt, R.W. Sample Preparation for Chromatography of Amino Acids: Acid Hydrolysis of Proteins. J. Assoc. Off. Anal. Chem. 1985, 68, 811–821. [Google Scholar] [CrossRef]
- Landry, J.; Delhaye, S. Simplified Procedure for the Determination of Tryptophan of Foods and Feedstuffs from Barytic Hydrolysis. J. Agric. Food Chem. 1992, 40, 776–779. [Google Scholar] [CrossRef]
- Aluko, R.E.; McIntosh, T. Electrophoretic and Functional Properties of Mustard Seed Meals and Protein Concentrates. J. Am. Oil Chem. Soc. 2004, 81, 679. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Malomo, S.A.; Fagbemi, T.N.; Aluko, R.E. Polypeptide Composition and Functional Properties of African Yam Bean Seed (Sphenostylis stenocarpa) Albumin, Globulin and Protein Concentrate. Food Hydrocoll. 2016, 56, 189–200. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Agboola, S.O.; Aluko, R.E. Isolation and Structural Properties of the Major Protein Fraction in Australian Wattle Seed (Acacia victoriae Bentham). Food Chem. 2009, 115, 1187–1193. [Google Scholar] [CrossRef]
- Lobley, A.; Whitmore, L.; Wallace, B.A. Dichroweb: An interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics 2002, 18, 211–212. [Google Scholar] [CrossRef] [Green Version]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [Green Version]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Chao, D.; Jung, S.; Aluko, R.E. Physicochemical and Functional Properties of High Pressure-Treated Isolated Pea Protein. Innov. Food Sci. Emerg. Technol. 2018, 45, 179–185. [Google Scholar] [CrossRef]
- Hsu, H.W.; Vavak, D.L.; Satterlee, D.L.; Mill, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The Effects of Limited Enzymatic Hydrolysis on the Physicochemical and Emulsifying Properties of a Lentil Protein Isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S. Physicochemical and Functional Properties of Lentil Protein Isolates Prepared by Different Drying Methods. Food Chem. 2011, 129, 1513–1522. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. In Vitro Protein Digestibility and Physico-Chemical Properties of Flours and Protein Concentrates from Two Varieties of Lentil (Lens Culinaris). Food Funct. 2013, 4, 310–321. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and Digestibility of Albumins and Globulins from Lentil and Horse Gram and Their Effect on Starch Rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Arise, A.K.; Nwachukwu, I.D.; Aluko, R.E.; Amonsou, E.O. Structure, Composition and Functional Properties of Storage Proteins Extracted from Bambara Groundnut (Vigna subterranea) Landraces. Int. J. Food Sci. Technol. 2017, 52, 1211–1220. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Wang, N.; Daun, J.K. Effects of Variety and Crude Protein Content on Nutrients and Anti-Nutrients in Lentils (Lens culinaris). Food Chem. 2006, 95, 493–502. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Chapter 16-Amino Acid Metabolism. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: New York, NY, USA; pp. 367–399. ISBN 978-0-12-803550-4.
- Gómez, M.; Oliete, B.; Rosell, C.M.; Pando, V.; Fernández, E. Studies on Cake Quality Made of Wheat–Chickpea Flour Blends. LWT-Food Sci. Technol. 2008, 41, 1701–1709. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-Chain Amino Acids as Pharmacological Nutrients in Chronic Liver Disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Meletis, C.D.; Barker, J.E. Therapeutic Uses of Amino Acids. Altern. Complementary Ther. 2005, 11, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Uneyama, H.; Kobayashi, H.; Tonouchi, N. New Functions and Potential Applications of Amino Acids. In Amino Acid Fermentation; Yokota, A., Ikeda, M., Eds.; Springer Japan: Tokyo, Japan, 2017; ISBN 978-4-431-56520-8. [Google Scholar]
- El-Beltagi, H.S.; El-Senousi, N.A.; Ali, Z.A.; Omran, A.A. The Impact of Using Chickpea Flour and Dried Carp Fish Powder on Pizza Quality. PLoS ONE 2017, 12, e0183657. [Google Scholar] [CrossRef] [Green Version]
- Grela, E.R.; Kiczorowska, B.; Samolińska, W.; Matras, J.; Kiczorowski, P.; Rybiński, W.; Hanczakowska, E. Chemical Composition of Leguminous Seeds: Part I—Content of Basic Nutrients, Amino Acids, Phytochemical Compounds, and Antioxidant Activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Sotak-Peper, K.M.; González-Vega, J.C.; Stein, H.H. Amino Acid Digestibility in Soybean Meal Sourced from Different Regions of the United States and Fed to Pigs1. J. Anim. Sci. 2017, 95, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Pirman, T.; Stibilj, V.; Stekar, J.M.; Combe, E. Amino Acid Composition of Beans and Lentil. Zb Bioteh Fak Univ Ljubl Kmet Zooteh 2001, 78, 57–68. [Google Scholar]
- Zia-Ul-Haq, M.; Ahmad, S.; Shad, M.; Iqbal, S.; Qayum, M.; Ahmad, A.; Luthria, D.; Amarowicz, R. Compositional Studies of Lentil (Lens Culinaris Medik.) Cultivars Commonly Grown in Pakistan. Pak. J. Bot. 2011, 43, 1563–1567. [Google Scholar]
- Berrazaga, I.; Bourlieu-Lacanal, C.; Laleg, K.; Jardin, J.; Briard-Bion, V.; Dupont, D.; Walrand, S.; Micard, V. Effect of Protein Aggregation in Wheat-Legume Mixed Pasta Diets on Their in Vitro Digestion Kinetics in Comparison to “Rapid” and “Slow” Animal Proteins. PLoS ONE 2020, 15, e0232425. [Google Scholar] [CrossRef]
- Singhal, A.; Karaca, A.C.; Tyler, R.; Nickerson, M. Pulse Proteins: From Processing to Structure-Function Relationships. Grain Legumes 2016, 55. [Google Scholar] [CrossRef] [Green Version]
- Jarpa-Parra, M. Lentil Protein: A Review of Functional Properties and Food Application. An Overview of Lentil Protein Functionality. Int. J. Food Sci. Technol. 2018, 53, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Aryee, A.N.A.; Boye, J.I. Improving the Digestibility of Lentil Flours and Protein Isolate and Characterization of Their Enzymatically Prepared Hydrolysates. Int. J. Food Prop. 2016, 19, 2649–2665. [Google Scholar] [CrossRef]
- Joehnke, M.S.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Sørensen, J.C.; Petersen, I.L. Nutritional and Anti-Nutritional Properties of Lentil (Lens culinaris) Protein Isolates Prepared by Pilot-Scale Processing. Food Chem. X 2021, 9, 100112. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of Limited Enzymatic Hydrolysis on the Structure and Emulsifying Properties of Rice Bran Protein. J. Cereal Sci. 2019, 85, 168–174. [Google Scholar] [CrossRef]
- Schmid, F.X. Protein Structure: A Practical Approach, Spectral Methods of Characterizing Protein Conformation and Conformational Changes; IRL Press: Oxford, UK, 1989; pp. 251–285. [Google Scholar]
- Eftink, M.R. Intrinsic Fluorescence of Proteins. In Topics in Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2000; Volume 6, pp. 1–15. ISBN 978-0-306-47102-5. [Google Scholar]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to Study Proteins by Circular Dichroism. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A.; Aguilera, Y.; Pedrosa, M.M.; Cuadrado, C.; Hernández, T.; Díaz, S.; Esteban, R.M. The Impact of Dehydration Process on Antinutrients and Protein Digestibility of Some Legume Flours. Food Chem. 2009, 114, 1063–1068. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between Digestibility and Secondary Structure of Raw and Thermally Treated Legume Proteins: A Fourier Transform Infrared (FT-IR) Spectroscopic Study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Elsohaimy, S.A.; Refaay, T.M.; Zaytoun, M.A.M. Physicochemical and Functional Properties of Quinoa Protein Isolate. Ann. Agric. Sci. 2015, 60, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Marambe, H.K.; Shand, P.J.; Wanasundara, J.P.D. In Vitro Digestibility of Flaxseed (Linum usitatissimum L.) Protein: Effect of Seed Mucilage, Oil and Thermal Processing. Int. J. Food Sci. Technol. 2013, 48, 628–635. [Google Scholar] [CrossRef]
- Arogundade, L.A.; Mu, T.-H.; Akinhanmi, T.F. Structural, Physicochemical and Interfacial Stabilisation Properties of Ultrafiltered African Yam Bean (Sphenostylis stenocarpa) Protein Isolate Compared with Those of Isoelectric Protein Isolate. LWT-Food Sci. Technol. 2016, 69, 400–408. [Google Scholar] [CrossRef]
- Jahaniaval, F.; Kakuda, Y.; Abraham, V.; Marcone, M.F. Soluble Protein Fractions from pH and Heat Treated Sodium Caseinate: Physicochemical and Functional Properties. Food Res. Int. 2000, 33, 637–647. [Google Scholar] [CrossRef]
- Mine, Y. Effect of Dry Heat and Mild Alkaline Treatment on Functional Properties of Egg White Proteins. J. Agric. Food Chem. 1997, 45, 2924–2928. [Google Scholar] [CrossRef]
- Rahmati, N.F.; Koocheki, A.; Varidi, M.; Kadkhodaee, R. Introducing Speckled Sugar Bean (Phaseolus vulgaris) Protein Isolates as a New Source of Emulsifying Agent. Food Hydrocoll. 2018, 79, 498–508. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the Functional Properties of Pea, Chickpea and Lentil Protein Concentrates Processed Using Ultrafiltration and Isoelectric Precipitation Techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. A Comparative Study of the Structural and Functional Properties of Isolated Hemp Seed (Cannabis sativa L.) Albumin and Globulin Fractions. Food Hydrocoll. 2015, 43, 743–752. [Google Scholar] [CrossRef]
- Damodaran, S. Food Proteins and Their Applications; CRC Press: New York, NY, USA, 2017; ISBN 978-1-351-44753-9. [Google Scholar]
- Aydemir, L.Y.; Yemenicioğlu, A. Potential of Turkish Kabuli Type Chickpea and Green and Red Lentil Cultivars as Source of Soy and Animal Origin Functional Protein Alternatives. LWT-Food Sci. Technol. 2013, 50, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of PH on the Inter-Relationships between the Physicochemical, Interfacial and Emulsifying Properties for Pea, Soy, Lentil and Canola Protein Isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and Functional Characterization of Kidney Bean and Field Pea Protein Isolates: A Comparative Study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal Processing Effects on the Functional Properties and Microstructure of Lentil, Chickpea, and Pea Flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L. Optimization of Lentil Protein Extraction and the Influence of Process PH on Protein Structure and Functionality. LWT-Food Sci. Technol. 2014, 57, 461–469. [Google Scholar] [CrossRef]










| DEF | ISO | MEM_NaOH | MEM_NaCl | |
|---|---|---|---|---|
| Moisture content (%) | 7.12 ± 0.54 a | 5.24 ± 0.72 b | 8.59 ± 0.52 a | 2.64 ± 0.83 c |
| Crude protein (%) | 29.35 ± 0.45 d | 86.13 ± 1.02 b | 82.55 ± 0.54 c | 90.28 ± 0.67 a |
| Crude fibre (%) | 4.41 ± 0.25 a | 0.10 ± 0.07 b | 0.02 ± 0.02 c | 0.02 ± 0.01 c |
| Fat (%) | 1.36 ± 0.66 b | 0.35 ± 0.13 d | 1.57 ± 0.23 a | 0.39 ± 0.15 c |
| Ash (%) | 2.96 ± 0.04 d | 6.00 ± 0.21 a | 3.72 ± 0.17 c | 4.75 ± 0.05 b |
| Non-Fibre Carbohydrates (%) | 60.99 ± 1.32 a | 6.48 ± 1.43 c | 11.24 ± 0.95 b | 3.61 ± 0.76 c |
| AA | ISO | MEM_NaOH | MEM_NaCl | Amino Acid Score (%) |
|---|---|---|---|---|
| Asx | 12.29 ± 0.17 | 12.89 ± 0.12 | 11.79 ± 0.38 | |
| Thr 1 | 3.78 ± 0.00 | 4.06 ± 0.02 | 4.76 ± 0.06 | 118 a, 108 b, 140 c |
| Ser | 5.40 ± 0.08 | 5.38 ± 0.14 | 5.12 ± 0.09 | |
| Glx | 18.01 ± 0.19 | 17.71 ± 0.09 | 15.77 ± 0.11 | |
| Pro | 4.19 ± 0.06 | 4.69 ± 0.00 | 4.21 ± 0.04 | |
| Gly | 3.47 ± 0.13 | 3.79 ± 0.04 | 4.02 ± 0.07 | |
| Ala | 3.84 ± 0.06 | 3.85 ± 0.09 | 4.84 ± 0.19 | |
| Cys 1 | 0.96 ± 0.56 | 1.41 ± 0.13 | 1.38 ± 0.13 | 163 a, 153 b, 164 c |
| Val 1 | 5.21 ± 0.10 | 5.33 ± 0.01 | 5.49 ± 0.05 | 95 a, 83 b, 94 c |
| Met 1 | 1.01 ± 0.04 | 1.31 ± 0.06 | 1.33 ± 0.02 | 47 a, 52 b, 56 c |
| Ile 1 | 4.52 ± 0.42 | 4.78 ± 0.49 | 4.26 ± 0.41 | 115 a, 104 b, 102 c |
| Leu 1 | 8.43 ± 0.04 | 8.18 ± 0.09 | 7.31 ± 0.05 | 103 a, 85 b, 83 c |
| Tyr | 3.55 ± 0.02 | 3.56 ± 0.16 | 4.18 ± 0.40 | |
| Phe | 5.81 ± 0.06 | 5.74 ± 0.02 | 5.08 ± 0.02 | |
| His 1 | 2.32 ± 0.25 | 2.41 ± 0.35 | 2.36 ± 0.21 | 120 a, 108 b, 112 c |
| Lys 1 | 6.75 ± 0.18 | 6.54 ± 0.13 | 8.78 ± 0.39 | 110 a, 90 b, 135 c |
| Arg | 8.94 ± 0.25 | 6.92 ± 0.11 | 6.58 ± 0.06 | |
| Trp 1 | 0.85 ± 0.06 | 1.01 ± 0.05 | 1.20 ± 0.03 | 107 a, 106 b, 136 c |
| AAA | 10.21 ± 0.01 | 10.31 ± 0.10 | 10.46 ± 0.40 | |
| BCAA | 18.16 ± 0.37 | 18.30 ± 0.60 | 17.06 ± 0.41 | |
| HAA | 38.38 ± 0.95 | 39.87 ± 0.78 | 39.28 ± 0.32 | |
| PCAA | 18.01 ± 0.68 | 15.87 ± 0.59 | 17.72 ± 0.54 | |
| NCAA | 30.30 ± 0.02 | 30.61 ± 0.21 | 27.56 ± 0.49 | |
| SCAA | 1.97 ± 0.60 | 2.72 ± 0.19 | 2.70 ± 0.15 |
| ISO | ||||
| pH | 3 | 5 | 7 | 9 |
| α-helix | 2.37 ± 0.03 | 2.52 ± 0.03 | 7.65 ± 0.28 | 6.05 ± 0.28 |
| β-sheet | 17.60 ± 0.07 | 17.00 ± 0.14 | 9.75 ± 0.64 | 12.38 ± 0.39 |
| Turns | 18.90 ± 0.00 | 18.85 ± 0.21 | 14.20 ± 0.28 | 13.65 ± 0.64 |
| Unordered | 41.25 ± 0.07 | 42.10 ± 0.14 | 50.95 ± 1.48 | 49.55 ± 1.34 |
| Total | 100.10 ± 0.00 | 100.00 ± 0.00 | 99.95 ± 0.07 | 100.05 ± 0.07 |
| 100.10 ± 0.28 | 100.00 ± 0.07 | 99.95 ± 3.61 | 100.05 ± 3.32 | |
| MEM_NaOH | ||||
| pH | 3 | 5 | 7 | 9 |
| α-helix | 2.45 ± 0.00 | 3.35 ± 0.14 | 3.92 ± 0.25 | 5.65 ± 0.64 |
| β-sheet | 17.57 ± 0.03 | 14.87 ± 0.39 | 13.55 ± 0.64 | 12.00 ± 0.21 |
| Turns | 18.50 ± 0.00 | 17.80 ± 0.28 | 16.80 ± 1.27 | 14.40 ± 0.28 |
| Unordered | 41.50 ± 0.00 | 45.75 ± 1.20 | 48.25 ± 2.19 | 50.30 ± 0.14 |
| Total | 100.05 ± 0.07 | 100.00 ± 0.14 | 100.00 ± 0.14 | 100.00 ± 0.00 |
| 100.05 ± 0.07 | 100.00 ± 2.54 | 100.00 ± 5.23 | 100.00 ± 2.12 | |
| MEM_NaCl | ||||
| pH | 3 | 5 | 7 | 9 |
| α-helix | 6.20 ± 0.28 | 7.78 ± 0.60 | 5.08 ± 0.25 | 6.13 ± 0.32 |
| β-sheet | 11.85 ± 0.85 | 13.25 ± 0.28 | 13.17 ± 0.39 | 12.20 ± 0.49 |
| Turns | 17.40 ± 0.42 | 17.95 ± 0.35 | 13.35 ± 0.92 | 12.55 ± 0.49 |
| Unordered | 46.50 ± 0.71 | 40.00 ± 2.12 | 50.20 ± 1.27 | 50.75 ± 0.21 |
| Total | 100 ± 0.00 | 100.00 ± 0.00 | 100.05 ± 0.07 | 99.95 ± 0.07 |
| 100 ± 3.39 | 100.00 ± 4.24 | 100.05 ± 3.46 | 99.95 ± 2.33 | |
| Sample | Onset To (°C) | Maximum Tp (°C) | Area ∆H (J/g of Sample) |
|---|---|---|---|
| ISO | 86.43 ± 0.49 a | 90.66 ± 0.10 | 0.16 ± 0.09 b |
| MEM_NaOH | 86.19 ± 0.13 b | 90.72 ± 0.12 | 0.19 ± 0.04 b |
| MEM_NaCl | 85.86 ± 0.34 c | 90.90 ± 0.06 a | 0.34 ± 0.00 a |
| ISO | MEM_NaOH | MEM_NaCl | |
|---|---|---|---|
| Protein yield | 48.45 ± 0.76 a | 35.05 ± 1.64 b | 13.35 ± 0.05 c |
| Protein digestibility | 89.82 ± 0.13 a | 77.97 ± 0.5 b | 77.61 ± 0.00 b |
| LGC | 11.50 ± 0.71 a | 11 ± 0.00 a | 10.75 ± 0.35 a |
| Surface hydrophobicity | 5.14 ± 0.11 b | 1.06 ± 0.02 c | 5.80 ± 0.01 a |
| WHC (40 mg/mL) | 0.15 ± 0.05 b | 0.47 ± 0.67 a | 0.10 ± 0.08 b |
| OHC (40 mg/mL) | 1.63 ± 1.97 a | 1.07 ± 1.48 b | 0.92 ± 1.21 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osemwota, E.C.; Alashi, A.M.; Aluko, R.E. Comparative Study of the Structural and Functional Properties of Membrane-Isolated and Isoelectric pH Precipitated Green Lentil Seed Protein Isolates. Membranes 2021, 11, 694. https://doi.org/10.3390/membranes11090694
Osemwota EC, Alashi AM, Aluko RE. Comparative Study of the Structural and Functional Properties of Membrane-Isolated and Isoelectric pH Precipitated Green Lentil Seed Protein Isolates. Membranes. 2021; 11(9):694. https://doi.org/10.3390/membranes11090694
Chicago/Turabian StyleOsemwota, Etinosa C., Adeola M. Alashi, and Rotimi E. Aluko. 2021. "Comparative Study of the Structural and Functional Properties of Membrane-Isolated and Isoelectric pH Precipitated Green Lentil Seed Protein Isolates" Membranes 11, no. 9: 694. https://doi.org/10.3390/membranes11090694
APA StyleOsemwota, E. C., Alashi, A. M., & Aluko, R. E. (2021). Comparative Study of the Structural and Functional Properties of Membrane-Isolated and Isoelectric pH Precipitated Green Lentil Seed Protein Isolates. Membranes, 11(9), 694. https://doi.org/10.3390/membranes11090694