Nanofiltration of the Remaining Whey after Kefir Grains’ Cultivation
Abstract
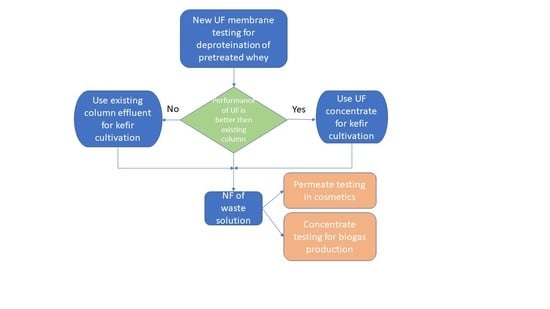
:1. Introduction
2. Materials and Methods
2.1. Filtration Setup and Procedure
2.2. Zeta Potential Measurements
2.3. SEM Imaging
2.4. NF Membrane Characterisation
2.5. Chemical Analyses
2.6. Microbiological Analyses
2.7. Spray-Drying of the Permeate
2.8. Anaerobic Fermentation
2.9. Calculations
3. Results and Discussion
3.1. Filtration with a UF Membrane
3.2. UF Membrane Cleaning
3.3. Filtration with an NF Membrane
3.3.1. Chemical Analyses
3.3.2. Microbiological Analyses
3.3.3. Experiments with a Moisturizing Solution
3.3.4. Experiments with Concentrate
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karim, A.; Aider, M. Comprehensive utilisation of electro-activated whey-based media in cell growth, metabolite production and aroma compounds synthesis using a starter culture originated from kefir grains. Int. Dairy J. 2022, 126, 105276. [Google Scholar] [CrossRef]
- Gogoi, M.; Biswas, T.; Biswal, P.; Saha, T.; Modak, A.; Gantayet, L.M.; Nath, R.; Mukherjee, I.; Thakur, A.R.; Sudarshan, M.; et al. A novel strategy for microbiological conversion of dairy wastewater into biofertilizer. J. Clean. Prod. 2021, 293, 126051. [Google Scholar] [CrossRef]
- López, E.R.T.; Leira, R.D.; Martínez, M.G.; Bugallo, P.M.B. Integrated environmental permit through best available techniques: Evaluation of the dairy industry. J. Clean. Prod. 2017, 162, 512–528. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Radchabut, S. Use of whey lactose from dairy industry for economical kefiran production by Lactobacillus kefiranofaciens in mixed cultures with yeasts. New Biotechnol. 2011, 28, 574–580. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sharma, H.; Melekoglu, E.; Ozogul, F. Recent developments in dairy kefir-derived lactic acid bacteria and their health benefits. Food Biosci. 2022, 46, 101592. [Google Scholar] [CrossRef]
- González-Orozco, B.D.; García-Cano, I.; Jiménez-Flores, F.; Alvárez, V.B. Invited review: Milk kefir microbiota—Direct and indirect antimicrobial effects. J. Dairy Sci. 2022, 105, 3703–3715. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Liu, J.R.; Sheu, J.F.; Lin, C.W.; Chuang, C.L. Study on skin care properties of milk kefir Whey, Asian-Australasian. J. Anim. Sci. 2006, 19, 905–908. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2021, 289, 132867. [Google Scholar] [CrossRef]
- Deng, H. A review on the application of ozonation to NF/RO concentrate for municipal wastewater reclamation. J. Hazard. Mater. 2020, 391, 122071. [Google Scholar] [CrossRef]
- Charalambous, P.; Shin, J.; Shin, S.G.; Vyrides, L. Anaerobic digestion of industrial dairy wastewater and cheese whey: Performance of internal circulation bioreactor and laboratory batch test at pH 5–6. Renew. Energy 2020, 147, 1–10. [Google Scholar] [CrossRef]
- Escalante, H.; Castro, L.; Amaya, M.P.; James, L.; James-Estevez, J. Anaerobic digestion of cheese whey: Energetic and nutrial potential for the dairy sector in developing countries. J. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Fino, D.; Erriquens, F.; Mancini, G.; Ruggeri, B. Scaled-up experimental biogas production from two agro-food waste mixtures having high inhibitory compound concentrations. Renew. Energy 2015, 81, 71–77. [Google Scholar] [CrossRef]
- Maragkaki, A.E.; Vasileiadis, I.; Fountoulakis, M.; Kyriakou, A.; Lasaridi, K.; Manios, T. Improving biogas production from anaerobic co-digestion of sewage sludge with a thermal dried mixture of food waste, cheese whey and olive mill wastewater. J. Waste Manag. 2018, 71, 644–651. [Google Scholar] [CrossRef]
- Simonic, M.; Novak, Z.P. Study of whey Acid Whey Fouling after Protein Isolation using Nanofiltration. Membranes 2021, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Regulation (2007), Decree on the Emission of Substances and Heat in the Discharge of Waste Water from Plants for the Treatment and Processing of Animal and Plant Raw Materials and Milk in the Food Production for Human Consumption and Animal Feed, Official Gazette of Republic of Slovenia, No 45/07, 44/22-ZVO-2. Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=URED4451 (accessed on 7 October 2022).
- Zirngast, K.; Petrovič, A.; Podričnik, M.; Čuček, L. Synthesis of biogas supply network based on experimental data from lab-scale anaerobic digestion of sewage sludge and organic waste. Chem. Eng. Trans. 2021, 88, 1051–1056. [Google Scholar] [CrossRef]
- Steinhauer, T.; Schwing, J.; Krauß, S.; Kulozik, U. Enhancement of ultrafiltration-performance and improvement of hygienic quality during the production of whey concentrates. Int. Dairy J. 2015, 45, 8–14. [Google Scholar] [CrossRef]
- Rani, S.R.S.; Kumar, V. Insight on applications of low-cost ceramic membranes in wastewater treatment: A mini-review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- D’Souza, N.M.; Mawson, A.J. Membrane Cleaning in the Dairy Industry: A Review. Crit. Rev. Food Sci. Nutr. 2005, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Corbaton-Baguena, M.J.; Alvarez-Blanco, S.; Vincent-Vela, M.C. Fouling mechanisms of ultrafiltration membranes fouled with whey model solutions. Desalination 2015, 360, 87–96. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Gierycz, P. Scaling of nanofiltration membranes used for chromium(III) ions recovery from salt solution. Water Sci. Technol. 2016, 76, wst2017456. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Duke, M.C.; Gray, S.R.; Weeks, M.; Palmer, M.; Vasiljevic, T. Strategies for maximizing removal of lactic acid from acid whey—Addressing the un-processability issue. Sep. Purif. Technol. 2017, 172, 489–497. [Google Scholar] [CrossRef]
- Chourasia, R.; Kumari, R.; Singh, S.P.; Sahoo, D.; Rai, A.K. Characterization of native lactic acid bacteria from traditionally fermented chhurpi of Sikkim Himalayan region for the production of chhurpi cheese with enhanced antioxidant effect. LWT 2022, 154, 112801. [Google Scholar] [CrossRef]
- Jasko, J.; Skripsts, E.; Dubrovskis, V.; Zabarovskis, E.; Kotelenecs, V. Biogas production from cheesy whey in two phase anaerobic digestion. In Proceedings of the Conference: 10th International scientific conference “Engineering for rural development”, Jelgava, Latvia, 26–27 May 2011. [Google Scholar]
- Marchetti, R.; Vasmara, C.; Bertin, L.; Fiume, F. Conversion of waste cooking oil into biogas: Perspectives and limits. Appl. Microbiol. Biotechnol. 2020, 104, 2833–2856. [Google Scholar] [CrossRef]
- Petrovič, A.; Zirngast, K.; Predikaka, T.C.; Simonič, M.; Cucek, L. The advantages of co-digestion of vegetable oil industry byproducts and sewage sludge: Biogas production potential. J. Environ. Manag. 2022, 318, 115566. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, R.; Wang, E.; Guo, J.; Zhou, Y.; Dong, R. Coupling biorefinery and biogas production from maize stover by enhancing the ensiling process: Role of the carbon/nitrogen ratio and buffer capacity. J. Clean. Prod. 2022, 339, 130770. [Google Scholar] [CrossRef]









| Parameter | UF | NF |
|---|---|---|
| Producer | Jiuwu Ceramic membrane | Suez |
| P | 0.5–10 bar | 0.5–28 bar |
| Tmax | 100 °C | 50 °C |
| pH | 0–14 | 2–11 |
| Pore size | 50 nm | 10 nm |
| Morphology | Ceramic multichannel α-Al2O3/ZrO2 | Thin film poly(piperazineamide) |
| Parameter | Units | Standard Method |
|---|---|---|
| w (protein) | g/100 g | ISO 8968/IDF 30-3 (2004) |
| w (lactose) | g/100 g | ISO 22662/IDF 198 (2007) |
| w (fat) | g/100 g | ISO 1211/IDF 1 (2010) |
| DM | g/100 g | ISO 6731/IDF 21 (2010, mod.) |
| w (Lactic acid) | mg/100 g | Boeringer Mannheim/R-Biopharm kit, 2021 |
| w (ash) | g/100 g | AOAC 938.08, gravimetric |
| w (Ca) | g/100 g | AOAC 938.08, gravimetric |
| w (K) | g/100 g | AOAC 938.08, gravimetric |
| w (Fe) pH | mg/100 g - | AOAC 938.08, gravimetric ISO 10523 (1996) |
| Parameter | Standard Method |
|---|---|
| E.coli | ISO 16649-2 (2001) |
| Coliforms | ISO 4832 (2006) |
| Listeria monocytogenes | ISO 11290-1 (2017) |
| Salmonella spp. | ISO 6579-1 (2017) |
| Total number of microorganisms | EN ISO 4833-1 (2013) |
| Staflococcus aureus | ISO 6888-2 (1999) |
| Spectrum | O | Al | Ca | Zr |
|---|---|---|---|---|
| 1 | 51.82 | 10.97 | 1.64 | 35.56 |
| 2 | 51.30 | 10.7 | 0.68 | 37.32 |
| 3 | 30.97 | 1.00 | - | 68.03 |
| 4 | 30.86 | 1.00 | - | 68.14 |
| PARAMETER | RW | Permeate | Rejection (%) |
|---|---|---|---|
| w (proteins) (g/100 g) | 0.69 | 0.04 | 94 |
| w (lactose) (g/100 g) | 0.428 | 0.0 | 100 |
| w (fat) (g/100 g) | 0 | 0 | - |
| DM (g/100 g) | 6.02 | 0.38 | 94 |
| pH | 3.3 | 3.1 | 3.2 |
| w (lactic acid) (mg/100 g) | 661.1 | 316.4 | 50 |
| w (ash) (g/100 g) | 0.921 | 0.171 | 81 |
| w (Ca) (g/100 g) | 0.108 | 0.051 | 53 |
| w (K) (g/100 g) | 0.186 | 0.051 | 73 |
| w (Fe) (mg/100 g) | 0.120 | 0.034 | 72 |
| PARAMETER | RW | Permeate | Concentrate |
|---|---|---|---|
| E. coli | <1 CFU/mL | <1 CFU/mL | <1 CFU/mL |
| Coliforms | <1 CFU/mL | <1 CFU/mL | <1 CFU/mL |
| Listeria monocytogenes | ND * | ND | ND |
| Salmonella spp. | ND | ND | ND |
| Total number of microorganisms | 1600 CFU/mL | 9.4 × 105 CFU/mL | 1.1 × 106 CFU/mL |
| Staphylococcus aureus | <10 CFU/mL | <10 CFU/mL | <10 CFU/mL |
| Day/Substrate | 5 | 10 Biogas (mL/(gVS)) | 20 | 30 |
|---|---|---|---|---|
| SVOI | 558 | 1260 | 1513 | 1630 |
| RW concentrate | 340 | 597 | 640 | 650 |
| SVOI and RW concentrate | 450 | 930 | 1054 | 1132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonič, M. Nanofiltration of the Remaining Whey after Kefir Grains’ Cultivation. Membranes 2022, 12, 993. https://doi.org/10.3390/membranes12100993
Simonič M. Nanofiltration of the Remaining Whey after Kefir Grains’ Cultivation. Membranes. 2022; 12(10):993. https://doi.org/10.3390/membranes12100993
Chicago/Turabian StyleSimonič, Marjana. 2022. "Nanofiltration of the Remaining Whey after Kefir Grains’ Cultivation" Membranes 12, no. 10: 993. https://doi.org/10.3390/membranes12100993
APA StyleSimonič, M. (2022). Nanofiltration of the Remaining Whey after Kefir Grains’ Cultivation. Membranes, 12(10), 993. https://doi.org/10.3390/membranes12100993