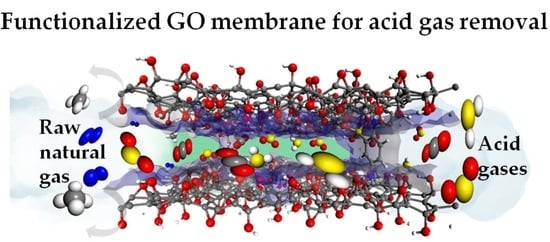
Functionalized GO Membranes for Efficient Separation of Acid Gases from Natural Gas: A Computational Mechanistic Understanding
Abstract
:1. Introduction
2. Models and Methods
3. Results and Discussion
3.1. Adsorption Evaluation
3.2. Adsorption Insight
3.3. Diffusion Evaluation
3.4. Separation Performance Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbols | |
| Qex, Qab | excess, absolute adsorption capacity (mmol·g−1) |
| ρg | gas density (mmol·cm−3) |
| Vf, Vo | free, occupied volumes (cm3·g−1) per unit mass |
| δ, β and γ | fitting parameters in the adsorption model |
| QL | maximal adsorption capacity in the Langmuir model |
| KL | adsorption equilibrium constant in the Langmuir model |
| n | empirical constant in the Redlich-Peterson model |
| R2 | correlation coefficient |
| g(r) | radial distribution function (RDF) |
| MSD | mean square displacement (nm2) |
| AFV | accessible free volume (%) |
| Si | solubility coefficient (cm3(STP)·cm−3·mmHg) |
| Di | diffusion coefficient (10−7 cm2·s−1) |
| Pi | permeability coefficient (Barrer) |
| αi/j, | gas selectivity of species i over j |
References
- Plant, G.; Kort, E.A.; Brandt, A.R.; Chen, Y.; Fordice, G.; Negron, A.M.G.; Schwietzke, S.; Smith, M.; Zavala-Araiza, D. Inefficient and unlit natural gas flares both emit large quantities of methane. Science 2022, 377, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, J.-T.; Wang, Y.; Yang, R.T. CO2 capture (including direct air capture) and natural gas desulfurization of amine-grafted hierarchical bimodal silica. Chem. Eng. J. 2021, 427, 131561. [Google Scholar] [CrossRef]
- Park, J.; Yoon, S.; Oh, S.-Y.; Kim, Y.; Kim, J.-K. Improving energy efficiency for a low-temperature CO2 separation process in natural gas processing. Energy 2020, 214, 118844. [Google Scholar] [CrossRef]
- Behmadi, R.; Mokhtarian, M.; Davoodi, A.; Hosseinpour, S. Desulfurization of natural gas condensate using polyethylene glycol and water intercalated activated γ-bauxite. J. Clean. Prod. 2022, 376, 134230. [Google Scholar] [CrossRef]
- He, Z.; Gupta, K.M.; Linga, P.; Jiang, J. Molecular Insights into the Nucleation and Growth of CH4 and CO2 Mixed Hydrates from Microsecond Simulations. J. Phys. Chem. C 2016, 120, 25225–25236. [Google Scholar] [CrossRef]
- He, Z.; Mi, F.; Ning, F. Molecular insights into CO2 hydrate formation in the presence of hydrophilic and hydrophobic solid surfaces. Energy 2021, 234, 121260. [Google Scholar] [CrossRef]
- He, Z.; Linga, P.; Jiang, J. What are the key factors governing the nucleation of CO2 hydrate? Phys. Chem. Chem. Phys. 2017, 19, 15657–15661. [Google Scholar] [CrossRef]
- Cavaignac, R.S.; Ferreira, N.L.; Guardani, R. Techno-economic and environmental process evaluation of biogas upgrading via amine scrubbing. Renew. Energy 2021, 171, 868–880. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Zhao, D. Mixed Matrix Membranes for Natural Gas Upgrading: Current Status and Opportunities. Ind. Eng. Chem. Res. 2018, 57, 4139–4169. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Jin, W. Natural gas purification by asymmetric membranes: An overview. Green Energy Environ. 2020, 6, 176–192. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Guo, Z.; Almansour, F.; Holmes, S.M.; Budd, P.M.; Gorgojo, P. Porous silica nanosheets in PIM-1 membranes for CO2 separation. J. Membr. Sci. 2022, 661, 120889. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Polymeric membranes for CO2 separation and capture. J. Membr. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; Téllez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar] [CrossRef]
- Liu, G.; Cadiau, A.; Liu, Y.; Adil, K.; Chernikova, V.; Carja, I.-D.; Belmabkhout, Y.; Karunakaran, M.; Shekhah, O.; Zhang, C.; et al. Enabling Fluorinated MOF-Based Membranes for Simultaneous Removal of H2S and CO2 from Natural Gas. Angew. Chem. 2018, 130, 15027–15032. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Varghese, A.M.; Reinalda, D.; Karanikolos, G.N. Graphene-based membranes for carbon dioxide separation. J. CO2 Util. 2021, 49, 101544. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Mao, Y.; Liu, G. Theoretical study on Janus graphene oxide membrane for water transport. Front. Chem. Sci. Eng. 2020, 15, 913–921. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, R.; Ye, Q.; Chen, B.; Zhu, X. Multilayered graphene oxide membrane with precisely controlled interlayer spacing for separation of molecules with very close molecular weights. J. Membr. Sci. 2022, 657, 120678. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Q.; Zhao, J.; Guan, K.; Mao, Y.; Liu, G.; Gu, X.; Jin, W. Ultrafast water-selective permeation through graphene oxide membrane with water transport promoters. AIChE J. 2019, 66, e16812. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Ameen, A.W.; Leaper, S.; Skuse, C.; Almansour, F.; Budd, P.M.; Gorgojo, P. PIM-1 membranes containing—Graphene oxide for CO2 separation. Sep. Purif. Technol. 2022, 298, 121447. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, C.; Wu, L.-g.; Shen, J.-n.; Van der Bruggen, B.; Chen, Q.; Chen, D.; Dong, C.-y. Fabrication of polyimide membrane incorporated with functional graphene oxide for CO2 separation: The effects of GO surface modification on membrane performance. Environ. Sci. Technol. 2017, 51, 6202–6210. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, C.; Do-Thanh, C.-L.; Dai, S. Two-Dimensional Materials as Prospective Scaffolds for Mixed-Matrix Membrane-Based CO2 Separation. ChemSusChem 2017, 10, 3304–3316. [Google Scholar] [CrossRef]
- Yan, F.; Guo, Y.; Wang, Z.; Zhao, L.; Zhang, X. Efficient separation of CO2/CH4 by ionic liquids confined in graphene oxide: A molecular dynamics simulation. Sep. Purif. Technol. 2022, 289, 120736. [Google Scholar] [CrossRef]
- Khakpay, A.; Rahmani, F.; Nouranian, S.; Scovazzo, P. Molecular insights on the CH4/CO2 separation in nanoporous graphene and graphene oxide separation platforms: Adsorbents versus membranes. J. Phys. Chem. C 2017, 121, 12308–12320. [Google Scholar]
- Zheng, X.; Ban, S.; Liu, B.; Chen, G. Strain-controlled graphdiyne membrane for CO2/CH4 separation: First-principle and molecular dynamic simulation. Chin. J. Chem. Eng. 2020, 28, 1898–1903. [Google Scholar] [CrossRef]
- Kallo, M.T.; Lennox, M.J. Understanding CO2/CH4 separation in pristine and defective 2D MOF CuBDC nanosheets via nonequilibrium molecular dynamics. Langmuir 2020, 36, 13591–13600. [Google Scholar]
- Wang, Z.; Yan, F.; Bai, L.; Zhang, X.; Liu, X.; Zhang, X. Insight into CO2/CH4 separation performance in ionic liquids/polymer membrane from molecular dynamics simulation. J. Mol. Liq. 2022, 357, 119119. [Google Scholar]
- Liu, Q.; Zhu, H.; Liu, G.; Jin, W. Efficient separation of (C1–C2) alcohol solutions by graphyne membranes: A molecular simulation study. J. Membr. Sci. 2021, 644, 120139. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, L.; Liu, G. Enhanced Selective Hydrogen Permeation through Graphdiyne Membrane: A Theoretical Study. Membranes 2020, 10, 286. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Liu, G. Simulation of cations separation through charged porous graphene membrane. Chem. Phys. Lett. 2020, 753, 137606. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Wang, X.; Liu, G.; Zhu, Y.; Tu, Y.; Lu, X.; Jin, W. Molecular dynamics simulation of water-ethanol separation through monolayer graphene oxide membranes: Significant role of O/C ratio and pore size. Sep. Purif. Technol. 2019, 224, 219–226. [Google Scholar] [CrossRef]
- Liu, Q.; Gupta, K.M.; Xu, Q.; Liu, G.; Jin, W. Gas permeation through double-layer graphene oxide membranes: The role of interlayer distance and pore offset. Sep. Purif. Technol. 2018, 209, 419–425. [Google Scholar] [CrossRef]
- Siepmann, J.I.; Frenkel, D. Configurational bias Monte Carlo: A new sampling scheme for flexible chains. Mol. Phys. 1992, 75, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Braga, C.; Travis, K.P. A configurational temperature Nosé-Hoover thermostat. J. Chem. Phys. 2005, 123, 134101. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Pan, D.; Li, J.; Zhang, L.; Shao, X. Application of Berendsen barostat in dissipative particle dynamics for nonequilibrium dynamic simulation. J. Chem. Phys. 2017, 146, 124108. [Google Scholar] [CrossRef]
- Lopez-Echeverry, J.S.; Reif-Acherman, S.; Araujo-Lopez, E. Peng-Robinson equation of state: 40 years through cubics. Fluid Phase Equilib. 2017, 447, 39–71. [Google Scholar] [CrossRef]
- Chen, L.; Liu, K.; Jiang, S.; Huang, H.; Tan, J.; Zuo, L. Effect of adsorbed phase density on the correction of methane excess adsorption to absolute adsorption in shale. Chem. Eng. J. 2021, 420, 127678. [Google Scholar] [CrossRef]
- De Lorenzo, L.; Tocci, E.; Gugliuzza, A.; Drioli, E. Pure and modified co-poly(amide-12-b-ethylene oxide) membranes for gas separation studied by molecular investigations. Membranes 2012, 2, 346–366. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhu, X.; Hu, J.; Liu, H.; Hu, Y.; Jiang, J. Mechanistic insight into highly efficient gas permeation and separation in a shape-persistent ladder polymer membrane. Phys. Chem. Chem. Phys. 2014, 16, 6075–6083. [Google Scholar] [CrossRef]
- Tocci, E.; Gugliuzza, A.; De Lorenzo, L.; Macchione, M.; De Luca, G.; Drioli, E. Transport properties of a co-poly(amide-12-b-ethylene oxide) membrane: A comparative study between experimental and molecular modelling results. J. Membr. Sci. 2008, 323, 316–327. [Google Scholar] [CrossRef]
- Alafnan, S.; Awotunde, A.; Glatz, G.; Adjei, S.; Alrumaih, I.; Gowida, A. Langmuir adsorption isotherm in unconventional resources: Applicability and limitations. J. Pet. Sci. Eng. 2021, 207, 109172. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, H.; Shan, Y. Adsorption of fluoride on synthetic siderite from aqueous solution. J. Fluor. Chem. 2010, 131, 635–641. [Google Scholar] [CrossRef]
- Tocci, E.; De Lorenzo, L.; Bernardo, P.; Clarizia, G.; Bazzarelli, F.; Mckeown, N.B.; Carta, M.; Malpass-Evans, R.; Friess, K.; Pilnáček, K.; et al. Molecular Modeling and Gas Permeation Properties of a Polymer of Intrinsic Microporosity Composed of Ethanoanthracene and Tröger’s Base Units. Macromolecules 2014, 47, 7900–7916. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant adsorption isotherms: A review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef]
- Javanainen, M.; Hammaren, H.; Monticelli, L.; Jeon, J.-H.; Miettinen, M.S.; Martinez-Seara, H.; Metzler, R.; Vattulainen, I. Anomalous and normal diffusion of proteins and lipids in crowded lipid membranes. Faraday Discuss. 2012, 161, 397–417. [Google Scholar] [CrossRef]
- Mazo, M.; Khudobin, R.; Balabaev, N.; Belov, N.; Ryzhikh, V.; Nikiforov, R.; Chatterjee, R.; Banerjee, S. Structure and free volume of fluorine-containing polyetherimides with Pendant di-tert-butyl groups investigated by molecular dynamics simulation. Polymer 2022, 14, 125318. [Google Scholar] [CrossRef]
- Qian, Q.; Wright, A.M.; Lee, H.; Dincă, M.; Smith, Z.P. Low-temperature H2S/CO2/CH4 separation in mixed-matrix membranes containing MFU-4. Chem. Mater. 2021, 33, 6825–6831. [Google Scholar] [CrossRef]
- Liu, G.; Cadiau, A.; Liu, Y.; Adil, K.; Chernikova, V.; Carja, I.-D.; Belmabkhout, Y.; Karunakaran, M.; Shekhah, O.; Zhang, C.; et al. Enabling fluorinated MOF-based membranes for simultaneous removal of H2S and CO2 from natural gas. Angew. Chem. Int. Ed. 2018, 57, 14811–14816. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; Téllez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar] [CrossRef]
- Yahaya, G.O.; Hayek, A.; Alsamah, A.; Shalabi, Y.A.; Ben Sultan, M.M.; Alhajry, R.H. Copolyimide membranes with improved H2S/CH4 selectivity for high-pressure sour mixed-gas separation. Sep. Purif. Technol. 2021, 272, 118897. [Google Scholar] [CrossRef]
- Hayek, A.; Alsamah, A.; Alaslai, N.; Maab, H.; Qasem, E.A.; Alhajry, R.H.; Alyami, N.M. Unprecedented Sour Mixed-Gas Permeation Properties of Fluorinated Polyazole-Based Membranes. ACS Appl. Polym. Mater. 2020, 2, 2199–2210. [Google Scholar] [CrossRef]
- Hayek, A.; Yahaya, G.O.; Alsamah, A.; Alghannam, A.A.; Jutaily, S.A.; Mokhtari, I. Pure- and sour mixed-gas transport properties of 4,4′-methylenebis(2,6-diethylaniline)-based copolyimide membranes. Polymer 2019, 166, 184–195. [Google Scholar] [CrossRef]
- Hayek, A.; Alsamah, A.; Yahaya, G.O.; Qasem, E.A.; Alhajry, R.H. Post-synthetic modification of CARDO-based materials: Application in sour natural gas separation. J. Mater. Chem. A 2020, 8, 23354–23367. [Google Scholar] [CrossRef]
- Alghannam, A.A.; Yahaya, G.O.; Hayek, A.; Mokhtari, I.; Saleem, Q.; Sewdan, D.A.; Bahamdan, A.A. High pressure pure- and mixed sour gas transport properties of Cardo-type block co-polyimide membranes. J. Membr. Sci. 2018, 553, 32–42. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, G.; Qiu, W.; Bhuwania, N.; Chinn, D.; Koros, W.J. Surprising plasticization benefits in natural gas upgrading using polyimide membranes. J. Membr. Sci. 2020, 593, 117430. [Google Scholar] [CrossRef]
- Liu, G.; Chernikova, V.; Liu, Y.; Zhang, K.; Belmabkhout, Y.; Shekhah, O.; Zhang, C.; Yi, S.; Eddaoudi, M.; Koros, W.J. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat. Mater. 2018, 17, 283–289. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yang, Z.; Liu, G.; Sun, L.; Xu, R.; Zhong, J. Functionalized GO Membranes for Efficient Separation of Acid Gases from Natural Gas: A Computational Mechanistic Understanding. Membranes 2022, 12, 1155. https://doi.org/10.3390/membranes12111155
Liu Q, Yang Z, Liu G, Sun L, Xu R, Zhong J. Functionalized GO Membranes for Efficient Separation of Acid Gases from Natural Gas: A Computational Mechanistic Understanding. Membranes. 2022; 12(11):1155. https://doi.org/10.3390/membranes12111155
Chicago/Turabian StyleLiu, Quan, Zhonglian Yang, Gongping Liu, Longlong Sun, Rong Xu, and Jing Zhong. 2022. "Functionalized GO Membranes for Efficient Separation of Acid Gases from Natural Gas: A Computational Mechanistic Understanding" Membranes 12, no. 11: 1155. https://doi.org/10.3390/membranes12111155
APA StyleLiu, Q., Yang, Z., Liu, G., Sun, L., Xu, R., & Zhong, J. (2022). Functionalized GO Membranes for Efficient Separation of Acid Gases from Natural Gas: A Computational Mechanistic Understanding. Membranes, 12(11), 1155. https://doi.org/10.3390/membranes12111155