Design and Evaluation of Two-Stage Membrane-Separation Processes for Propylene–Propane Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Problem Statement
2.2. Membranes
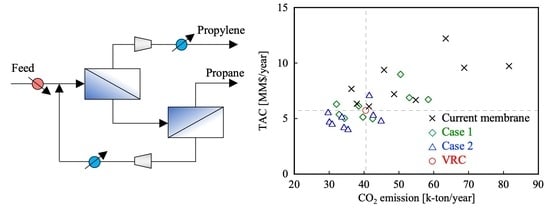
2.3. Membrane-Separation Process
2.4. Design and Evaluation Scheme
2.5. Distillation Benchmark
2.6. Evaluation of CO2 Emissions and TAC
- The payback (depreciation) period = 4 y.
- The annual operating time = 8000 h.
- The compressor is a single stage one.
- The compression efficiency = 0.75.
- The overall heat transfer coefficient = 0.671 kW/(m2 K) [44].
- The price of current membrane = 500 $/m2 [20].
- The price of the Case 1 membrane = 1000 $/m2.
- The price of the Case 2 membrane = 2000 $/m2.
3. Simulation Results and Discussion
3.1. Current Membranes
3.2. Case Studies of Technological Forecasts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Membrane | Permeate-Side Pressure [kPa] | Membrane Area [m2] | Energy Consumption [GJ/h] | CO2 Emission [k-ton/y] | |||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | M1 | M2 | ||
| A | A | 800 | 300 | 15,377 | 17,731 | 66.1 | 36.4 |
| A | B | 700 | 300 | 14,194 | 6860 | 69.4 | 38.0 |
| A | C | 600 | 300 | 13,180 | 2929 | 76.9 | 41.4 |
| B | A | 600 | 400 | 5398 | 33,307 | 86.2 | 45.8 |
| B | B | 600 | 400 | 5398 | 13,427 | 92.4 | 48.7 |
| B | C | 500 | 300 | 5038 | 4296 | 105.7 | 54.9 |
| C | A | 500 | 400 | 2073 | 43,893 | 124.1 | 63.4 |
| C | B | 400 | 400 | 1944 | 18,289 | 135.5 | 68.8 |
| C | C | 300 | 400 | 1829 | 8755 | 163.1 | 81.6 |
References
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain if chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Tanizume, S.; Yoshimura, T.; Ishii, K.; Nomura, M. Control of sequential MTO reactions through an MFI-type zeolite membrane contactor. Membranes 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Cat. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Ebadzadeh, E.; Khademi, M.H.; Beheshti, M. A kinetic model for methanol-to-propylene process in the presence of co-feeding of C4-C5 olefine mixture over H-ZSM-5 catalysis. Chem. Eng. J. 2021, 405, 126605. [Google Scholar] [CrossRef]
- Vajda, S.; Pellin, M.J.; Greeley, J.P.; Marshall, C.L.; Curtiss, L.A.; Ballentine, G.A.; Elam, J.W.; Catillon-Mucherie, S.; Redfern, P.C.; Mehmood, F.; et al. Subnanometer platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8, 213–216. [Google Scholar] [CrossRef]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New trends in olefin production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Chen, L.; Ye, Q.; Jiang, Z.; Yuan, J.; Zhang, H.; Wang, N. Novel methodology for determining the optimal vapor recompressed assisted distillation process based on economic and energy efficiency. Sep. Purif. Technol. 2020, 251, 117393. [Google Scholar] [CrossRef]
- Christopher, C.C.E.; Dutta, A.; Farooq, S.; Karimi, I.A. Process synthesis and optimization of propylene/propane separation using vapor recompression and self-heat recuperation. Ind. Eng. Chem. Res. 2017, 56, 14557–14564. [Google Scholar] [CrossRef]
- Li, H.; Cong, H.; Li, X.; Li, X.; Gao, X. Systematic design of the integration heat pump into heat integrated distillation column for recovering energy. Appl. Therm. Eng. 2016, 105, 93–104. [Google Scholar] [CrossRef]
- Alcántara-Avila, J.R.; Gómez-Castro, F.I.; Segovia-Hernández, J.G.; Sotowa, K.; Horikawa, T. Optimal design of cryogenic distillation columns with side heat pumps for the propylene/propane separation. Chem. Eng. Process. 2014, 82, 112–122. [Google Scholar] [CrossRef]
- Kumar, V.; Anand, A.; Kaistha, N. Design and control of a vapour recompression c3 splitter. Chem. Eng. Res. Des. 2020, 159, 410–423. [Google Scholar] [CrossRef]
- Grande, C.A.; Poplow, F.; Rodrigues, A.E. Vacuum pressure swing adsorption to produce polymer-grade propylene. Sep. Sci. Technol. 2010, 45, 1252–1259. [Google Scholar] [CrossRef]
- Abedini, H.; Asgari, M.; Watt Coull, M.; Shariati, A.; Reza Khosravi-Nikou, M. Efficient production of polymer-grade propylene from the propane/propylene binary mixture using Cu-MOF-74 framework. Sep. Purif. Technol. 2021, 276, 119172. [Google Scholar] [CrossRef]
- Sen, T.; Kawajiri, Y.; Realff, M.J. Adsorption process intensification through structured packing: A modeling study using zeolite 13X and a mixture of propylene and propane in hollow-fiber and packed Beds. Ind. Eng. Chem. Res. 2019, 58, 5750–5767. [Google Scholar] [CrossRef]
- Dobladez, J.A.D.; Maté, V.I. Á; Torrellas, S.Á; Larriba, M. Separation of the propane propylene mixture with high recovery by a dual PSA process. Comput. Chem. Eng. 2020, 136, 106717. [Google Scholar] [CrossRef]
- Kim, J.J.; Hong, S.H.; Park, D.; Chung, K.; Lee, C.H. Separation of propane and propylene by desorbent swing adsorption using zeolite 13X and carbon dioxide. Chem. Eng. J. 2021, 410, 128276. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Lee, U.; Kim, J.; Seok Chae, I.S.; Han, C. Techno-economic feasibility study of membrane based propane/propylene separation process. Chem. Eng. Process. 2017, 119, 62–72. [Google Scholar] [CrossRef]
- Zarca, R.; Ortiz, A.; Gorri, D.; Biegler, L.T.; Ortiz, I. Optimization of multistage olefin/paraffin membrane separation processes through rigorous modeling. AIChE J. 2019, 65, e16588. [Google Scholar] [CrossRef]
- Alcheikhhamdoh, Y.; Pinnau, I.; Hoorfar, M.; Chem, B. Propylene–propane separation using zeolitic-imidazolate framework (ZIF-8) Membranes: Process techno-commercial evaluation. J. Membr. Sci. 2019, 591, 117252. [Google Scholar] [CrossRef] [Green Version]
- Richard, W.B. Membrane Technology and Applications, 3rd ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Pressly, T.G.; Ng, K.M. A break-even analysis of distillation-membrane hybrids. AIChE J. 1998, 44, 93–105. [Google Scholar] [CrossRef]
- Zarca, R.; Ortiz, A.; Gorri, D.; Biegler, L.T.; Ortiz, I. Optimized distillation coupled with state-of-the-art membranes for propylene purification. J. Membr. Sci. 2018, 556, 321–328. [Google Scholar] [CrossRef]
- Yamaki, T.; Yoshimune, M.; Hara, N.; Negishi, H. Heat-integrated hybrid membrane separation-distillation process for energy-efficient isopropyl alcohol dehydration. J. Chem. Eng. Jpn. 2018, 51, 890–897. [Google Scholar] [CrossRef]
- Tula, A.K.; Befort, B.; Garg, N.; Camarda, K.V.; Gani, R. Sustainable process design & analysis of hybrid separations. Comput. Chem. Eng. 2017, 105, 96–104. [Google Scholar]
- Amedi, H.R.; Aghajani, M. Economic estimation of various membranes and distillation for propylene and propane separation. Ind. Eng. Chem. Res. 2018, 57, 4366–4376. [Google Scholar] [CrossRef]
- Benali, M.; Aydin, B. Ethane/Ethylene and Propane/Propylene Separation in Hybrid Membrane Distillation Systems: Optimization and Economic Analysis. Sep. Purif. Technol. 2010, 73, 377–390. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Brayden, M.K.; Martinez, M.V.; Stears, B.A.; Barbay, G.A.; Koros, W.J. Olefins-selective asymmetric carbon molecular sieve hollow fiber membranes for hybrid membrane—Distillation processes for olefin/paraffin separations. J. Membr. Sci. 2012, 423–424, 314–323. [Google Scholar] [CrossRef]
- Yamaki, T.; Yoshimune, M.; Hara, N.; Negishi, H. Energy-saving performance of membrane separation and hybrid membrane separation distillation for propylene/propane binary systems. J. Jpn. Petrol. Inst. 2019, 62, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Ma, X.; Lin, B.K.; Wei, X.; Kniep, J.; Lin, Y.S. Gamma-alumina supported carbon molecular sieve membrane for propylene/propane separation. Ind. Eng. Chem. Res. 2013, 52, 4297–4305. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.S.; Wei, X.; Kniep, J. Ultrathin Carbon Molecular Sieve Membrane for Propylene/Propane Separation. AIChE J. 2016, 62, 491–499. [Google Scholar] [CrossRef]
- Hayashi, J.; Mizuta, H.; Yamamoto, M.; Kusakabe, K.; Morooka, S.; Suh, S.H. Separation of ethane/ethylene and propane/propylene systems with a carbonized BPDA-pp’ODA polyimide membrane. Ind. Eng. Chem. Res. 1996, 35, 4176–4181. [Google Scholar] [CrossRef]
- Guo, M.; Kanezashi, M. Recent progress in a membrane-based technique for propylene/propane separation. Membrane 2021, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin Selective ag-exchange x-type zeolite membrane for propylene/propane and ethylene/ethane separation. A.C.S. Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar]
- Pan, Y.; Li, T.; Lestari, G.; Lai, Z. Effective separation of propylene/propane binary mixtures by ZIF-8 membranes. J. Membr. Sci. 2012, 390–391, 93–98. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Xi, H.; Lin, Y.S. Gas transport properties and propylene/propane separation characteristics of ZIF-8 membranes. J. Membr. Sci. 2014, 451, 85–93. [Google Scholar] [CrossRef]
- Tran, N.T.; Yu, T.; Kim, J.; Othman, M.R. ZIF-8 tubular membrane for propylene purification: Effect of surface curvature and zinc salts on separation performance. Sep. Purif. Technol. 2020, 251, 117354. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K. In site synthesis of thin zeolite-imidazolate framework zif-8 membranes exhibiting exceptionally high propylene/propane separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K. Improving propylene/propane separation performance of zeolitic-imidazolate framework zif-8 membranes. Chem. Eng. Sci. 2015, 124, 20–26. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. diffusive separation of propylene/propane with ZIF-8 membranes. J. Membr. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- IDEA Inventory Database for Environmental Analysis. I.D.E.A. Available online: http://tco2.com/app/com/page/IDEA.action?lc=en_US (accessed on 27 December 2021).
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A.; Bhattacharyya, D. Analysis, Synthesis, and Design of Chemical Processes, 4th ed.; Person Education Inc.: New York, NY, USA, 2013. [Google Scholar]
- Horiuchi, K.; Yanagimoto, K.; Kataoka, K.; Nakaiwa, M.; Iwakabe, K.; Matsuda, K. Energy saving characteristics of the internally heat integrated distillation colum (HIDiC) pilot plant for multicomponent petroleum distillation. J. Chem. Eng. Jpn. 2008, 41, 771–778. [Google Scholar] [CrossRef]
- International Monetary Fund. World Economic Outlook, A Long and Difficult Ascent. 2020. OCT. Available online: https://www.imf.org/en/Publications/WEO/Issues/2020/09/30/world-economic-outlook-october-2020 (accessed on 27 December 2021).










| Category | Membrane | Propylene Permeance | Propane Permeance | Separation Factor | ||
|---|---|---|---|---|---|---|
| [mol/(m2 s Pa)] | [GPU] * | [mol/(m2 s Pa)] | [GPU] * | [–] | ||
| Current membrane | A | 2.80 × 10−8 | 84 | 4.59 × 10−10 | 1.4 | 61 |
| B | 6.10 × 10−8 | 182 | 1.85 × 10−9 | 5.5 | 33 | |
| C | 1.38 × 10−7 | 411 | 8.12 × 10−9 | 24 | 17 | |
| Case 1 | D | 8.10 × 10−8 | 241 | 8.80 × 10−10 | 2.6 | 92 |
| E | 1.75 × 10−7 | 522 | 3.57 × 10−9 | 10 | 49 | |
| F | 3.90 × 10−7 | 1164 | 1.56 × 10−8 | 46 | 25 | |
| Case 2 | G | 2.30 × 10−7 | 686 | 1.69 × 10−9 | 5.0 | 136 |
| H | 5.00 × 10−7 | 1492 | 6.85 × 10−9 | 20 | 73 | |
| I | 1.10 × 10−6 | 3283 | 2.89 × 10−8 | 86 | 38 | |
| Parameters | Value |
|---|---|
| Feed temperature (K) | 322 |
| Feed pressure (kPa) | 2000 |
| Feed flow rate (kmol/h) | 1589 |
| Feed composition (propylene mole%) | 90 |
| Membrane feed-side pressure (kPa) | 2000 |
| Membrane temperature (K) [35] | 373 |
| Propylene purity (mol%) | 99.5 |
| Propylene recovery ratio (%) | 99.5 |
| Parameters | Value |
|---|---|
| Feed temperature (K) | 322 |
| Feed pressure (kPa) | 2000 |
| Feed flow rate (kmol/h) | 1589 |
| Feed composition (propylene mol%) | 90 |
| Feed stage a | 114 |
| Total number of stages b | 176 |
| Pressure at the top of the column (kPa) | 2000 |
| Reflux ratio | 15.9 |
| Condenser duty (GJ/h) | 288 |
| Reboiler duty (GJ/h) | 269 |
| Propylene purity (mol%) | 99.5 |
| Propylene recovery ratio (%) | 99.5 |
| Utility | CO2 Emissions [kg/GJ] | Cost [$/GJ] [43] |
|---|---|---|
| Steam | 172 | 14.2 |
| Electricity | 161 | 16.8 |
| Refrigerated water | 54 | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaki, T.; Thuy, N.; Hara, N.; Taniguchi, S.; Kataoka, S. Design and Evaluation of Two-Stage Membrane-Separation Processes for Propylene–Propane Mixtures. Membranes 2022, 12, 163. https://doi.org/10.3390/membranes12020163
Yamaki T, Thuy N, Hara N, Taniguchi S, Kataoka S. Design and Evaluation of Two-Stage Membrane-Separation Processes for Propylene–Propane Mixtures. Membranes. 2022; 12(2):163. https://doi.org/10.3390/membranes12020163
Chicago/Turabian StyleYamaki, Takehiro, Nguyen Thuy, Nobuo Hara, Satoshi Taniguchi, and Sho Kataoka. 2022. "Design and Evaluation of Two-Stage Membrane-Separation Processes for Propylene–Propane Mixtures" Membranes 12, no. 2: 163. https://doi.org/10.3390/membranes12020163
APA StyleYamaki, T., Thuy, N., Hara, N., Taniguchi, S., & Kataoka, S. (2022). Design and Evaluation of Two-Stage Membrane-Separation Processes for Propylene–Propane Mixtures. Membranes, 12(2), 163. https://doi.org/10.3390/membranes12020163