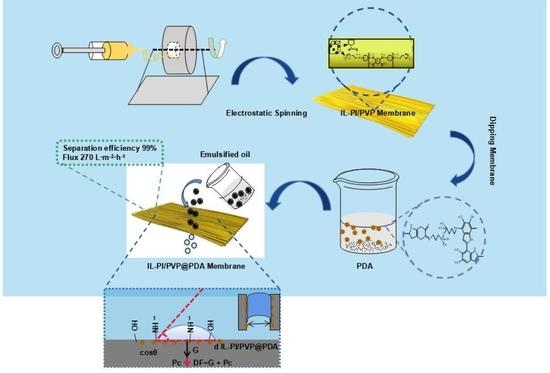
Preparation and Separation Properties of Electrospinning Modified Membrane with Ionic Liquid Terminating Polyimide/Polyvinylpyrrolidone@Polydopamine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Polymerization
2.3. Membrane Formation and Thermal Treatments
2.4. Coating Film Preparation
2.5. Emulsion Oil Preparation
2.6. Membrane Characterization
3. Results and Discussion
3.1. SEM Observation of Polyimide Membranes
3.2. Characteristics of Modified Membranes
3.3. Mechanical Properties of Membranes
3.4. Wettability of Membrane
3.5. Oil-Water Separation Performance of Membrane
3.6. Recyclability of IL-PI/PVP@PDA Fibrous Membrane
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Yong, J.; Huo, J.; Chen, F.; Yang, Q.; Hou, X. Oil/water separation based on natural materials with super-wettability: Recent advances. Phys. Chem. Chem. Phys. 2018, 20, 25140–25163. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Al-Kayiem, H.H.; Aleem, W.; Saad, A.B. Influence of alkali-surfactant-polymer flooding on the coalescence and sedimentation of oil/water emulsion in gravity separation. J. Pet. Sci. Eng. 2019, 173, 640–649. [Google Scholar] [CrossRef]
- Gou, X.; Zhang, Y.; Long, L.; Liu, Y.; Tian, D.; Shen, F.; Yang, G.; Zhang, X.; Wang, L.; Deng, S. Superhydrophilic and underwater superoleophobic cement-coated mesh for oil/water separation by gravity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125338. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Azevedo, A.; Rubio, J. Separation of emulsified crude oil in saline water by dissolved air flotation with micro and nanobubbles. Sep. Purif. Technol. 2017, 186, 326–332. [Google Scholar] [CrossRef]
- Al-Shamrani, A.; James, A.; Xiao, H. Separation of oil from water by dissolved air flotation. Colloids Surfaces A: Physicochem. Eng. Asp. 2002, 209, 15–26. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, D.; Xu, Y.; Zhao, S. Design and Computational Fluid Dynamics Analysis of a Three-Phase Decanter Centrifuge for Oil-Water-Solid Separation. Chem. Eng. Technol. 2020, 43, 1005–1015. [Google Scholar] [CrossRef]
- Guerrini, L.; Pantani, O.L.; Parenti, A. The impact of vertical centrifugation on olive oil quality. J. Food Process Eng. 2017, 40, e12489. [Google Scholar] [CrossRef]
- Antes, F.G.; Diehl, L.O.; Pereira, J.S.; Guimarães, R.C.; Guarnieri, R.A.; Ferreira, B.M.; Flores, E.M. Effect of ultrasonic frequency on separation of water from heavy crude oil emulsion using ultrasonic baths. Ultrason. Sonochem. 2017, 35, 541–546. [Google Scholar] [CrossRef]
- Chen, M.; Huang, L.; Liu, Z.; Liu, J.; Xing, Y.; Liu, X.; Jin, Z.; Wang, X. Development of superhydrophilic Al foil with micropore arrays via mask electrochemical machining and chemical immersion for efficient oil/water separation. J. Dispers. Sci. Technol. 2019, 41, 1335–1345. [Google Scholar] [CrossRef]
- Mousavi, S.-M.; Nejad, Z.M.; Hashemi, S.A.; Salari, M.; Gholami, A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W. Bioactive Agent-Loaded Electrospun Nanofiber Membranes for Accelerating Healing Process: A Review. Membranes 2021, 11, 702. [Google Scholar] [CrossRef]
- Kertik, A.; Wee, L.H.; Pfannmöller, M.; Bals, S.; Martens, J.A.; Vankelecom, I.F.J. Highly selective gas separation membrane using in situ amorphised metal–organic frameworks. Energy Environ. Sci. 2017, 10, 2342–2351. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Song, J.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Electrospun flexible nanofibrous membranes for oil/water separation. J. Mater. Chem. A 2019, 7, 20075–20102. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially Wettable Membranes for Oil–Water Separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]
- La, Y.-H.; McCloskey, B.; Sooriyakumaran, R.; Vora, A.; Freeman, B.; Nassar, M.; Hedrick, J.; Nelson, A.; Allen, R. Bifunctional hydrogel coatings for water purification membranes: Improved fouling resistance and antimicrobial activity. J. Membr. Sci. 2011, 372, 285–291. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A. On the way to clean and safe electrospinning-green electrospinning: Emulsion and suspension electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Yin, J.; Roso, M.; Boaretti, C.; Lorenzetti, A.; Martucci, A.; Modesti, M. PVDF-TiO2 core-shell fibrous membranes by microwave-hydrothermal method: Preparation, characterization, and photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 106250. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Sun, W.; Chen, J.; Dai, C.; Yan, B.; Zeng, H. Bio-inspired membrane with adaptable wettability for smart oil/water separation. J. Membr. Sci. 2020, 598, 117661. [Google Scholar] [CrossRef]
- Avval, Z.M.; Malekpour, L.; Raeisi, F.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Salari, M. Introduction of magnetic and supermagnetic nanoparticles in new approach of targeting drug delivery and cancer therapy application. Drug Metab. Rev. 2020, 52, 157–184. [Google Scholar] [CrossRef]
- Akhtar, S.; Hawes, C.; Dudley, L.; Reed, I.; Stratford, P. Coatings reduce the fouling of microfiltration membranes. J. Membr. Sci. 1995, 107, 209–218. [Google Scholar] [CrossRef]
- Du, Q.; Chen, Z.; Jiang, X.; Pang, J.; Jiang, Z.; Luan, J. An oil/water separation nanofibrous membrane with a 3-D structure from the blending of PES and SPEEK. High Perform. Polym. 2019, 31, 538–547. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Yan, L.; Bai, Y.; Li, S.; Sorokin, P.; Shao, L. Biomimetic nanoparticle-engineered superwettable membranes for efficient oil/water separation. J. Membr. Sci. 2021, 618, 118525. [Google Scholar] [CrossRef]
- Jiang, S.; Hou, H.; Agarwal, S.; Greiner, A. Polyimide Nanofibers by “Green” Electrospinning via Aqueous Solution for Filtration Applications. ACS Sustain. Chem. Eng. 2016, 4, 4797–4804. [Google Scholar] [CrossRef]
- Zhuo, L.; Ma, C.; Xie, F.; Chen, S.; Lu, Z. Methylcellulose strengthened polyimide aerogels with excellent oil/water separation performance. Cellul. 2020, 27, 7677–7689. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Dastageer, M. Polyimide based super-wettable membranes/materials for high performance oil/water mixture and emulsion separation: A review. Adv. Colloid Interface Sci. 2021, 297, 102525. [Google Scholar] [CrossRef]
- Lee, B.S.; Chi, Y.S.; Lee, J.K.; Choi, I.S.; Song, C.E.; Namgoong, S.K.; Lee, S.-G. Imidazolium Ion-Terminated Self-Assembled Monolayers on Au: Effects of Counteranions on Surface Wettability. J. Am. Chem. Soc. 2004, 126, 480–481. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, J.; Zhang, L.; Cheng, G.; Chen, B.; Zhang, Y.; Gao, G. Poly(ionic liquid)-Coated Meshes with Opposite Wettability for Continuous Oil/Water Separation. Ind. Eng. Chem. Res. 2020, 59, 6672–6680. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and Eumelanin: From Structure–Property Relationships to a Unified Tailoring Strategy. Accounts Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Sunab, F.; Li, T.-T.; Zhanga, X.; Shiuc, B.-C.; Zhanga, Y.; Rena, H.-T.; Penga, H.-K.; Linabcdefg, J.-H.; Louabcfhi, C.-W. In situ growth polydopamine decorated polypropylen melt-blown membrane for highly efficient oil/water separation. Chemosphere 2020, 254, 126873. [Google Scholar] [CrossRef]
- Pagidi, A.; Saranya, R.; Arthanareeswaran, G.; Ismail, A.; Matsuura, T. Enhanced oil–water separation using polysulfone membranes modified with polymeric additives. Desalination 2014, 344, 280–288. [Google Scholar] [CrossRef]
- Motta, A.; Borges, C.; Esquerre, K.; Kiperstok, A. Oil Produced Water treatment for oil removal by an integration of coalescer bed and microfiltration membrane processes. J. Membr. Sci. 2014, 469, 371–378. [Google Scholar] [CrossRef]












| Membrane | Basis Weight a (g/m2) | Pore Size b (μm) | Porosity c (%) |
|---|---|---|---|
| ODA-6FDA | 25.72 | 3.53 | 7.09 |
| IL-ODA-6FDA | 22.67 | 4.62 | 17.48 |
| IL-ODA-6FDA/PVP | 28.94 | 4.89 | 19.02 |
| IL-ODA-6FDA/PVP@PDA | 20.11 | 3.95 | 12.76 |
| No. | Designation | Εb b (%) | E c (MPa) | F d (L·m−2·h−1) | R e (%) | θ f (°) |
|---|---|---|---|---|---|---|
| 1 | ODA-6FDA | 8.90 ± 0.14 | 459.12 ± 0.22 | 120.27 ± 1.20 | 24.01 ± 6.01 | 128.97 ± 3.86 |
| 2 | IL-ODA-6FDA | 26.95 ± 0.44 | 2271.23 ± 1.01 | 172.45 ± 3.51 | 70.27 ± 8.62 | 114.70 ± 2.17 |
| 3 | IL-ODA-6FDA/PVP | 39.21 ± 0.31 | 3056.46 ± 1.26 | 161.31 ± 8.06 | 77.45 ± 3.87 | 64.43 ± 1.26 |
| 4 | IL-ODA-6FDA/PVP@PDA | 37.74 ± 0.47 | 3198.38 ± 0.78 | 270.40 ± 11.52 | 99.31 ± 0.16 | 30.26 ± 2.16 |
| 5 | MDA-BTDA | 20.50 ± 0.12 | 314.50 ± 0.09 | 139.63 ± 5.62 | 32.52 ± 7.92 | 103.50 ± 2.19 |
| 6 | IL-MDA-BTDA | 21.27 ± 0.36 | 1573.12 ± 1.30 | 150.74 ± 6.42 | 68.42 ± 5.63 | 87.65 ± 1.12 |
| 7 | IL-MDA-BTDA/PVP | 32.62 ± 0.52 | 2443.79 ± 1.26 | 190.59 ± 8.29 | 78.62 ± 7.94 | 54.73 ± 0.42 |
| 8 | IL-MDA-BTDA/PVP@PDA | 39.70 ± 0.43 | 3698.73 ± 0.96 | 204.74 ± 7.94 | 99.57 ± 0.23 | 29.02 ± 0.46 |
| 9 | ODA-PMDA | 2.84 ± 0.05 | 729.12 ± 0.17 | 148.74 ± 10.02 | 24.95 ± 6.96 | 74.10 ± 0.23 |
| 10 | IL-ODA-PMDA | 6.20 ± 0.03 | 3057.40 ± 0.70 | 136.79 ± 6.35 | 70.62 ± 5.53 | 56.85. ± 0.85 |
| 11 | IL-ODA-PMDA/PVP | 12.97 ± 0.32 | 3131.78 ± 0.16 | 176.83 ± 4.23 | 83.22 ± 3.97 | 54.23 ± 1.84 |
| 12 | IL-ODA-PMDA/PVP@PDA | 19.52 ± 0.02 | 3023.10 ± 1.24 | 286.41 ± 8.49 | 98.68 ± 0.21 | 18.16 ± 0.58 |
| 13 | MDA-BPDA | 14.35 ± 0.52 | 496.42 ± 1.58 | 105.83 ± 9.73 | 32.86 ± 8.84 | 136.43 ± 0.78 |
| 14 | IL-MDA-BPDA | 25.40 ± 0.07 | 1950.73 ± 1.91 | 164.72 ± 12.84 | 65.85 ± 6.29 | 82.52. ± 0.31 |
| 15 | IL-MDA-BPDA/PVP | 36.27 ± 0.06 | 2122.99 ± 1.06 | 210.58 ± 14.72 | 73.91 ± 3.42 | 64.40 ± 2.46 |
| 16 | IL-MDA-BPDA/PVP@PDA | 34.62 ± 0.26 | 2669.10 ± 1.84 | 283.82 ± 9.64 | 94.98 ± 1.83 | 21.11 ± 0.74 |
| 17 | ODA-BTDA | 2.20 ± 0.12 | 391.46 ± 0.09 | 135.85 ± 13.57 | 37.07 ± 5.97 | 96.30 ± 1.35 |
| 18 | IL-ODA-BTDA | 11.28 ± 0.03 | 2515.12 ± 1.30 | 182.97 ± 11.46 | 57.33 ± 8.31 | 76.49 ± 0.84 |
| 19 | IL-ODA-BTDA/PVP | 19.90 ± 0.05 | 2269.40 ± 0.17 | 205.63 ± 9.64 | 72.86 ± 4.31 | 69.64 ± 1.48 |
| 20 | IL-ODA-BTDA/PVP@PDA | 31.53 ± 0.03 | 3797.34 ± 0.70 | 263.24 ± 8.32 | 96.43 ± 1.97 | 15.31 ± 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, P.; Jia, H.; Xu, S.; Wang, Q.; Su, G.; Yang, G.; Zhang, M.; Qu, Y.; Pei, F. Preparation and Separation Properties of Electrospinning Modified Membrane with Ionic Liquid Terminating Polyimide/Polyvinylpyrrolidone@Polydopamine. Membranes 2022, 12, 189. https://doi.org/10.3390/membranes12020189
Qi P, Jia H, Xu S, Wang Q, Su G, Yang G, Zhang M, Qu Y, Pei F. Preparation and Separation Properties of Electrospinning Modified Membrane with Ionic Liquid Terminating Polyimide/Polyvinylpyrrolidone@Polydopamine. Membranes. 2022; 12(2):189. https://doi.org/10.3390/membranes12020189
Chicago/Turabian StyleQi, Peng, Hongge Jia, Shuangping Xu, Qingji Wang, Guiming Su, Guoxing Yang, Mingyu Zhang, Yanqing Qu, and Fuying Pei. 2022. "Preparation and Separation Properties of Electrospinning Modified Membrane with Ionic Liquid Terminating Polyimide/Polyvinylpyrrolidone@Polydopamine" Membranes 12, no. 2: 189. https://doi.org/10.3390/membranes12020189
APA StyleQi, P., Jia, H., Xu, S., Wang, Q., Su, G., Yang, G., Zhang, M., Qu, Y., & Pei, F. (2022). Preparation and Separation Properties of Electrospinning Modified Membrane with Ionic Liquid Terminating Polyimide/Polyvinylpyrrolidone@Polydopamine. Membranes, 12(2), 189. https://doi.org/10.3390/membranes12020189