High Methoxyl Pectin and Sodium Caseinate Film Matrix Reinforced with Green Carbon Quantum Dots: Rheological and Mechanical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instrumentation
2.2.1. Rheological Characterization
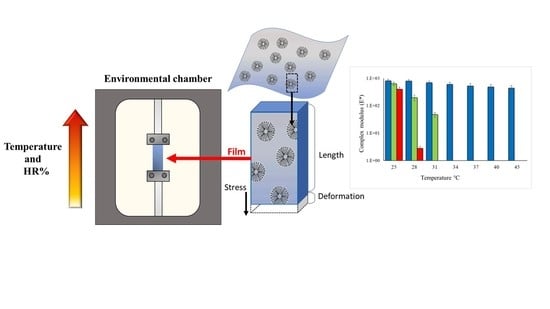
2.2.2. Mechanical Characterization
2.3. Preparation of the Sodium Caseinate and Pectin Suspensions
2.4. Synthesis of gCDs from Apple Pomace and Rosemary Leaves
Preparation of CAS/HMP Suspensions and Biopolymer Films Doped with gCDs
3. Results and Discussion
3.1. The Characterization of Green Carbon Dots
3.1.1. Morphological Characterization of the CAS/HMP Suspensions
3.1.2. Steady-State Shear Rate
3.2. Thermal Behavior
3.3. Rheological Characterization of CAS/HMP Suspensions Enriched with gCDs
3.4. Dopped CAS/HMP Biopolymer Films
Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalita, N.K.; Damare, N.A.; Hazarika, D.; Bhagabati, P.; Kalamdhad, A.; Katiyar, V. Biodegradation and characterization study of compostable PLA bioplastic containing algae biomass as potential degradation accelerator. Environ. Chall. 2021, 3, 100067. [Google Scholar] [CrossRef]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N. A Review of Bioplastics and Their Adoption in the Circular Economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Yarmand, M.S.; Mousavi, M.; Degraeve, P.; Oulahal, N.; Gharsallaoui, A. Complex coacervation for the development of composite edible films based on LM pectin and sodium caseinate. Carbohydr. Polym. 2016, 151, 947–956. [Google Scholar] [CrossRef]
- Ezati, P.; Roy, S.; Rhim, J.-W. Pectin/gelatin-based bioactive composite films reinforced with sulfur functionalized carbon dots. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 636, 128123. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Tu, H.; Zhang, H.; Silvester, D.S.; Banks, C.E.; Zou, G.; Hou, H.; Ji, X. The development of carbon dots: From the perspective of materials chemistry. Mater. Today 2021, 51, 188–207. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon dot/polymer nanocomposites: From green synthesis to energy, environmental and biomedical applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Miller, K.; Krochta, J. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Functional Biopolymer Particles: Design, Fabrication, and Applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.R.; Raphael, E.; Pawlicka, A. Plasticized pectin-based gel electrolytes. Electrochim. Acta 2009, 54, 6479–6483. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef] [Green Version]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Q.; Daubert, C.R.; Velev, O.D. Physicochemical Variables Affecting the Rheology and Microstructure of Rennet Casein Gels. J. Agric. Food Chem. 2007, 55, 2688–2697. [Google Scholar] [CrossRef]
- Jahromi, M.; Niakousari, M.; Golmakani, M.T.; Mohammadifar, M.A. Physicochemical and structural characterization of sodium caseinate based film-forming solutions and edible films as affected by high methoxyl pectin. Int. J. Biol. Macromol. 2020, 165, 1949–1959. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, S. Creating high yield water soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes. Chem. Commun. 2012, 48, 10177–10179. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Huang, Y.; Wang, X.; Zhang, X.; Wang, X. Adsorption, Aggregation, and Deposition Behaviors of Carbon Dots on Minerals. Environ. Sci. Technol. 2017, 51, 6156–6164. [Google Scholar] [CrossRef]
- Khan, M.B.; Khoker, M.F.; Husain, M.; Ahmed, M.; Anwer, S. Effects of Nanoparticles on Rheological Behavior of Polyacrylamide Related to Enhance Oil Recovery. Acad. J. Polym. Sci. 2018, 1, 555573. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, R.P. Non-Newtonian Fluids: An Introduction. In Rheology of Complex Fluids; Krishnan, J., Deshpande, A., Kumar, P., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Safaie, B.; Youssefi, M.; Rezaei, B. Rheological behavior of polypropylene/carbon quantum dot nanocomposites: The effects of particles size, particles/matrix interface adhesion, and particles loading. Polym. Bull. 2019, 76, 4335–4354. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Fragiadakis, D.; Pissis, P. Reinforcement effect of carbon nanofillers in an epoxy resin system: Rheology, molecular dynamics, and mechanical studies. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 522–533. [Google Scholar] [CrossRef]
- Arrigo, R.; Malucelli, G. Rheological Behavior of Polymer/Carbon Nanotube Composites: An Overview. Materials 2020, 13, 2771. [Google Scholar] [CrossRef]
- Mayorga, O.L.; Freire, E. Dynamic analysis of differential scanning calorimetry data. Biophys. Chem. 1987, 27, 87–96. [Google Scholar] [CrossRef]
- Roldughin, V.I.; Serenko, O.A.; Getmanova, E.V.; Novozhilova, N.A.; Nikifirova, G.G.; Buzin, M.I.; Chvalun, S.N.; Ozerin, A.N.; Muzafarov, A.M. Effect of hybrid nanoparticles on glass transition temperature of polymer nanocomposites. Polym. Compos. 2016, 37, 1978–1990. [Google Scholar] [CrossRef]
- Norton, I.T.; Spyropoulos, F.; Cox, P. (Eds.) Practical Food Rheology. An Interpretive Approach. In Chap. 2 Viscosity and Oscillatory Rheology by T. Miri; Wiley-Blackwell Publishing Ltd.: Oxford, UK, 2011. [Google Scholar]
- Zhuang, Y.; Sterr, J.; Kulozik, U.; Gebhardt, R. Application of confocal Raman microscopy to investigate casein micro-particles in blend casein/pectin films. Int. J. Biol. Macromol. 2015, 74, 44–48. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 4th ed.; Vincentz Network GmbH: Hannover, Germany, 2014; pp. 135–210. [Google Scholar]
- Babaee, M.; Garavand, F.; Rehman, A.; Jafarazadeh, S.; Amini, E.; Cacciotti, I. Biodegradability, physical, mechanical and antimicrobial attributes of starch nanocomposites containing chitosan nanoparticles. Int. J. Biol. Macromol. 2022, 195, 49–58. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chem. Commun. 2018, 54, 5401–5406. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murru, C.; Mohammadifar, M.A.; Wagner, J.B.; Badía Laiño, R.; Díaz García, M.E. High Methoxyl Pectin and Sodium Caseinate Film Matrix Reinforced with Green Carbon Quantum Dots: Rheological and Mechanical Studies. Membranes 2022, 12, 695. https://doi.org/10.3390/membranes12070695
Murru C, Mohammadifar MA, Wagner JB, Badía Laiño R, Díaz García ME. High Methoxyl Pectin and Sodium Caseinate Film Matrix Reinforced with Green Carbon Quantum Dots: Rheological and Mechanical Studies. Membranes. 2022; 12(7):695. https://doi.org/10.3390/membranes12070695
Chicago/Turabian StyleMurru, Clarissa, Mohammad Amin Mohammadifar, Jakob Birkedal Wagner, Rosana Badía Laiño, and Marta Elena Díaz García. 2022. "High Methoxyl Pectin and Sodium Caseinate Film Matrix Reinforced with Green Carbon Quantum Dots: Rheological and Mechanical Studies" Membranes 12, no. 7: 695. https://doi.org/10.3390/membranes12070695
APA StyleMurru, C., Mohammadifar, M. A., Wagner, J. B., Badía Laiño, R., & Díaz García, M. E. (2022). High Methoxyl Pectin and Sodium Caseinate Film Matrix Reinforced with Green Carbon Quantum Dots: Rheological and Mechanical Studies. Membranes, 12(7), 695. https://doi.org/10.3390/membranes12070695