Synthesis and Characterization of Silica–Tantala Microporous Membranes for Gas Separations Fabricated Using Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Synthesis
2.2. Membrane Characterization
3. Results and Discussion
3.1. Microstructure Analysis
3.2. Elemental Analysis
3.3. Effect of Increasing Tantalum Content
3.4. Thermal vs. Reactive Deposition
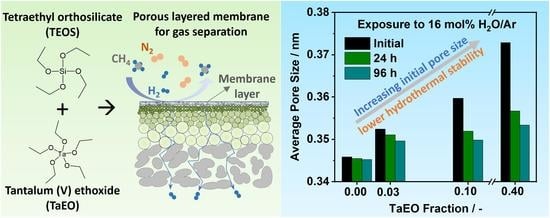
3.5. Hydrothermal Stability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Algieri, C.; Coppola, G.; Mukherjee, D.; Shammas, M.I.; Calabro, V.; Curcio, S.; Chakraborty, S. Catalytic membrane reactors: The industrial applications perspective. Catalysts 2021, 11, 691. [Google Scholar] [CrossRef]
- Julbe, A.; Farrusseng, D.; Guizard, C. Porous ceramic membranes for catalytic reactors—Overview and new ideas. J. Membr. Sci. 2001, 181, 3–20. [Google Scholar] [CrossRef]
- Jokar, S.M.; Farokhnia, A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Gonçalves, M.C.; Basile, A. The recent areas of applicability of palladium based membrane technologies for hydrogen production from methane and natural gas: A review. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Ovalle-Encinia, O.; Wu, H.-C.; Chen, T.; Lin, J.Y.S. CO2-permselective membrane reactor for steam reforming of methane. J. Membr. Sci. 2022, 641, 119914. [Google Scholar] [CrossRef]
- Oyama, S.T.; Zhang, X.; Lu, J.; Gu, Y.; Fujitani, T. Epoxidation of propylene with H2 and O2 in the explosive regime in a packed-bed catalytic membrane reactor. J. Catal. 2008, 257, 1–4. [Google Scholar] [CrossRef]
- Luo, H.; Wei, Y.; Jiang, H.; Yuan, W.; Lv, Y.; Caro, J.; Wang, H. Performance of a ceramic membrane reactor with high oxygen flux Ta-containing perovskite for the partial oxidation of methane to syngas. J. Membr. Sci. 2010, 350, 154–160. [Google Scholar] [CrossRef]
- Kniep, J.; Lin, Y.S. Partial oxidation of methane and oxygen permeation in SrCoFeOx membrane reactor with different catalysts. Ind. Eng. Chem. Res. 2011, 50, 7941–7948. [Google Scholar] [CrossRef]
- Aseem, A.; Harold, M.P. C2 yield enhancement during oxidative coupling of methane in a nonpermselective porous membrane reactor. Chem. Eng. Sci. 2018, 175, 199–207. [Google Scholar] [CrossRef]
- Bhatia, S.; Thien, C.Y.; Mohamed, A.R. Oxidative coupling of methane (OCM) in a catalytic membrane reactor and comparison of its performance with other catalytic reactors. Chem. Eng. J. 2009, 148, 525–532. [Google Scholar] [CrossRef]
- Yang, C.; Xu, N.; Shi, J. Experimental and modeling study on a packed-bed membrane reactor for partial oxidation of methane to formaldehyde. Ind. Eng. Chem. Res. 1998, 37, 2601–2610. [Google Scholar] [CrossRef]
- Lund, C.R.F. Improving selectivity during methane partial oxidation by use of a membrane reactor. Catal. Lett. 1992, 12, 395–403. [Google Scholar] [CrossRef]
- Cruellas, A.; Ververs, W.; Annaland, M.V.S.; Gallucci, F. Experimental investigation of the oxidative coupling of methane in a porous membrane reactor: Relevance of back-permeation. Membranes 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-based membranes for hydrogen separation: Review of alloying elements and their influence on membrane properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Ma, C.; Wang, M.; Wang, Z.; Gao, M.; Wang, J. Recent progress on thin film composite membranes for CO2 separation. J. CO2 Util. 2020, 42, 101296. [Google Scholar] [CrossRef]
- da Silva Biron, D.; dos Santos, V.; Zeni, M. Ceramic Membranes Applied in Separation Processes; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Yoshioka, T.; Nakata, A.; Tung, K.-L.; Kanezashi, M.; Tsuru, T. Molecular dynamics simulation study of solid vibration permeation in microporous amorphous silica network voids. Membranes 2019, 9, 132. [Google Scholar] [CrossRef]
- Ren, X.; Kanezashi, M.; Guo, M.; Xu, R.; Zhong, J.; Tsuru, T. Multiple amine-contained POSS-functionalized organosilica membranes for gas separation. Membranes 2021, 11, 194. [Google Scholar] [CrossRef]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Gavalas, G.R.; Megiris, C.E.; Nam, S.W. Deposition of H2-permselective SiO2 films. Chem. Eng. Sci. 1989, 44, 1829–1835. [Google Scholar] [CrossRef]
- Okubo, T.; Inoue, H. Introduction of specific gas selectivity to porous glass membranes by treatment with tetraethoxysilane. J. Membr. Sci. 1989, 42, 109–117. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Silica membranes for hydrogen separation prepared by chemical vapor deposition (CVD). Sep. Purif. Technol. 2013, 111, 20–42. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The sol–gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Oyama, S.T. High molecular permeance in a poreless ceramic membrane. Adv. Mater. 2007, 19, 1636–1640. [Google Scholar] [CrossRef]
- Oyama, S.T.; Yamada, M.; Sugawara, T.; Takagaki, A.; Kikuchi, R. Review on mechanisms of gas permeation through inorganic membranes. J. Jpn. Pet. Inst. 2011, 54, 298–309. [Google Scholar] [CrossRef]
- Mise, Y.; Ahn, S.-J.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Fabrication and evaluation of trimethylmethoxysilane (TMMOS)-derived membranes for gas separation. Membranes 2019, 9, 123. [Google Scholar] [CrossRef]
- Scholes, C.A. Helium recovery through inorganic membranes incorporated with a nitrogen rejection unit. Ind. Eng. Chem. Res. 2018, 57, 3792–3799. [Google Scholar] [CrossRef]
- Yang, X.; Du, H.; Lin, Y.; Song, L.; Zhang, Y.; Gao, X.; Kong, C.; Chen, L. Hybrid organosilica membrane with high CO2 permselectivity fabricated by a two-step hot coating method. J. Membr. Sci. 2016, 506, 31–37. [Google Scholar] [CrossRef]
- Kanezashi, M.; Matsutani, T.; Nagasawa, H.; Tsuru, T. Fluorine-induced microporous silica membranes: Dramatic improvement in hydrothermal stability and pore size controllability for highly permeable propylene/propane separation. J. Membr. Sci. 2018, 549, 111–119. [Google Scholar] [CrossRef]
- Wu, J.; Sabol, H.; Smith, G.; Flowers, D.; Liu, P. Characterization of hydrogen-permselective microporous ceramic membranes. J. Membr. Sci. 1994, 96, 275–287. [Google Scholar] [CrossRef]
- Gu, Y.; Hacarlioglu, P.; Oyama, S.T. Hydrothermally stable silica–alumina composite membranes for hydrogen separation. J. Membr. Sci. 2008, 310, 28–37. [Google Scholar] [CrossRef]
- Tsuru, T.; Tsuge, T.; Kubota, S.; Yoshida, K.; Yoshioka, T.; Asaeda, M. Catalytic membrane reaction for methane steam reforming using porous silica membranes. Sep. Sci. Technol. 2001, 36, 3721–3736. [Google Scholar] [CrossRef]
- Igi, R.; Yoshioka, T.; Ikuhara, Y.H.; Iwamoto, Y.; Tsuru, T. Characterization of Co-doped silica for improved hydrothermal stability and application to hydrogen separation membranes at high temperatures. J. Am. Ceram. Soc. 2008, 91, 2975–2981. [Google Scholar] [CrossRef]
- Asaeda, M.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Gas permeation characteristics and stability of composite silica-metal oxide membranes. Mater. Res. Soc. Symp. Proc. 2003, 752, 213–218. [Google Scholar] [CrossRef]
- Gu, Y.; Oyama, S.T. Permeation properties and hydrothermal stability of silica–titania membranes supported on porous alumina substrates. J. Membr. Sci. 2009, 345, 267–275. [Google Scholar] [CrossRef]
- Boffa, V.; Blank, D.H.; ten Elshof, J.E. Hydrothermal stability of microporous silica and niobia–silica membranes. J. Membr. Sci. 2008, 319, 256–263. [Google Scholar] [CrossRef]
- Wang, H.; Lundin, S.-T.B.; Takanabe, K.; Oyama, S.T. Synthesis of size-controlled boehmite sols: Application in high-performance hydrogen-selective ceramic membranes. J. Mater. Chem. A 2022, 10, 12869. [Google Scholar] [CrossRef]
- Fotou, G.P.; Lin, Y.S.; Pratsinis, S.E. Hydrothermal stability of pure and modified microporous silica membranes. J. Mater. Sci. 1995, 30, 2803–2808. [Google Scholar] [CrossRef]
- Kato, H.; Lundin, S.-T.B.; Ahn, S.-J.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Gas separation silica membranes prepared by chemical vapor deposition of methyl-substituted silanes. Membranes 2019, 9, 144. [Google Scholar] [CrossRef]
- Aono, H.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Synthesis of silica membranes by chemical vapor deposition using a dimethyldimethoxysilane precursor. Membranes 2020, 10, 50. [Google Scholar]
- Kageyama, N.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Synthesis and characterization of a silica-alumina compo-site membrane and its application in a membrane reactor. Sep. Purif. Technol. 2018, 195, 437–445. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirano, Y.; Fujii, H.; Tsuru, T.; Asaeda, M. Hydrothermal Stability and Performance of Silica-Zirconia Membranes for Hydrogen Separation in Hydrothermal Conditions. J. Chem. Eng. Jpn. 2001, 34, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.-J.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Permeation properties of silica-zirconia composite membranes supported on porous alumina substrates. J. Membr. Sci. 2017, 526, 409–416. [Google Scholar] [CrossRef]
- Tsuru, T.; Igi, R.; Kanezashi, M.; Yoshioka, T.; Fujisaki, S.; Iwamoto, Y. Permeation properties of hydrogen and water vapor through porous silica membranes at high temperatures. AIChE J. 2011, 57, 618–629. [Google Scholar] [CrossRef]
- Qureshi, H.F.; Besselink, R.; ten Elshof, J.E.; Nijmeijer, A.; Winnubst, L. Doped microporous hybrid silica membranes for gas separation. J. Sol-Gel Sci. Technol. 2015, 75, 180–188. [Google Scholar] [CrossRef]
- Suzuki, M.; Rohde, D. Resolving Overlapping Peak Problems with NORAN System 7 Spectral Imaging Software; Application Note 51188; Thermo Fischer Scientific: Madison, WI, USA, 2008; Available online: https://tools.thermofisher.com/content/sfs/brochures/D10051~.pdf (accessed on 15 August 2022).
- Seah, M.P.; Dench, W.A. Quantitative electron spectroscopy of surfaces: A standard database for electron inelastic mean free paths in solids. Surf. Interp. Anal. 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Brundle, C.R.; Castle, J.E.; Engelhard, M.H.; Gaskell, K.J.; Grant, J.T.; Haasch, R.T.; Linford, M.R.; Powell, C.J.; et al. Practical guides for X-ray photoelectron spectroscopy (XPS): First steps in planning, conducting and reporting XPS measurements. J. Vac. Sci. Technol. A 2019, 37, 031401. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin Elmer Corporation: Eden Prairie, MN, USA, 1992; p. 27. [Google Scholar]
- Lee, D.; Oyama, S.T. Gas permeation characteristics of a hydrogen selective supported silica membrane. J. Membr. Sci. 2002, 210, 291–306. [Google Scholar] [CrossRef]
- Hwang, G.-J.; Kim, J.-W.; Choi, H.-S.; Onuki, K. Stability of a silica membrane prepared by CVD using γ- and α-alumina tube as the support tube in the HI–H2O gaseous mixture. J. Membr. Sci. 2003, 215, 293–302. [Google Scholar] [CrossRef]
- Gao, X.; da Costa, J.C.D.; Bhatia, S.K. Adsorption and transport of gases in a supported microporous silica membrane. J. Membr. Sci. 2014, 460, 46–61. [Google Scholar] [CrossRef]
- Amanipour, M.; Babakhani, E.G.; Safekordi, A.; Zamaniyan, A.; Heidari, M. Effect of CVD parameters on hydrogen permeation properties in a nano-composite SiO2–Al2O3 membrane. J. Membr. Sci. 2012, 423–424, 530–535. [Google Scholar] [CrossRef]
- Duke, M.C.; Pas, S.J.; Hill, A.J.; Lin, Y.S.; da Costa, J.C.D. Exposing the molecular sieving architecture of amorphous silica using positron annihilation spectroscopy. Adv. Func. Mater. 2008, 18, 3818–3826. [Google Scholar] [CrossRef]
- Lee, H.R.; Kanezashi, M.; Shimomura, Y.; Yoshioka, T.; Tsuru, T. Evaluation and fabrication of pore-size-tuned silica membranes with tetraethoxydimethyl disiloxane for gas separation. AIChE J. 2010, 57, 2755–2765. [Google Scholar] [CrossRef]



 ], Si-3Ta-650 [
], Si-3Ta-650 [ ], Si-10Ta-650 [
], Si-10Ta-650 [ ], Si-40Ta-650 [
], Si-40Ta-650 [ ].
].
 ], Si-3Ta-650 [
], Si-3Ta-650 [ ], Si-10Ta-650 [
], Si-10Ta-650 [ ], Si-40Ta-650 [
], Si-40Ta-650 [ ].
].
 ], Si-3Ta-650 [
], Si-3Ta-650 [ ], Si-10Ta-650 [
], Si-10Ta-650 [ ], Si-40Ta-650 [
], Si-40Ta-650 [ ].
].
 ], Si-3Ta-650 [
], Si-3Ta-650 [ ], Si-10Ta-650 [
], Si-10Ta-650 [ ], Si-40Ta-650 [
], Si-40Ta-650 [ ].
].
 ], Si-10Ta-600 [
], Si-10Ta-600 [ ], Si-10Ta-500 [
], Si-10Ta-500 [ ], Si-10Ta-400 [
], Si-10Ta-400 [ ].
].
 ], Si-10Ta-600 [
], Si-10Ta-600 [ ], Si-10Ta-500 [
], Si-10Ta-500 [ ], Si-10Ta-400 [
], Si-10Ta-400 [ ].
].
 ], Si-3Ta-650 [
], Si-3Ta-650 [ ], Si-10Ta-650 [
], Si-10Ta-650 [ ], Si-40Ta-650 [
], Si-40Ta-650 [ ]. Permeance measurements taken during short interruptions of the steam exposure.
]. Permeance measurements taken during short interruptions of the steam exposure.
 ], Si-3Ta-650 [
], Si-3Ta-650 [ ], Si-10Ta-650 [
], Si-10Ta-650 [ ], Si-40Ta-650 [
], Si-40Ta-650 [ ]. Permeance measurements taken during short interruptions of the steam exposure.
]. Permeance measurements taken during short interruptions of the steam exposure.


| Membrane Material | Synthesis | H2O Exposure | H2 Permeance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Precursors * | Method | Temp. /°C | H2O Conc. /mol % | Time /h | Change /% | Final /mol m−2 s−1 Pa−1 | ||
| SiO2+Al2O3 | TEOS+ATSB | CVD | 650 | 16 | 100 | −45 | 3.9 × 10−8 | [40] |
| 650 | 16 | 96 | −68 | 2.3 × 10−8 | [40] | |||
| 600 | 16 | 200 | −39 | 1.3 × 10−7 | [30] | |||
| SiO2+ZrO2 | TEOS+ZTB | Sol–gel | 500 | 50 | 30 | −18 | 2.8 × 10−6 | [31] |
| Sol–gel | 500 | 13–33 | 30 | −73 | 1.1 × 10−7 | [41] | ||
| CVD | 650 | 16 | 48 | −56 | 1.7 × 10−7 | [42] | ||
| SiO2+CO3O4 | TEOS+ Co(NO3)2·6H2O | Sol–gel | 500 | 50 | 60 | −50 | 4.0 × 10−8 | [32] |
| 500 | 30 | 60 | −47 | 1.8 × 10−7 | [43] | |||
| SiO2+NiO | TEOS+ Ni(NO3)2·6H2O | Sol–gel | 40 | 4.4 | 1680 | −50 | 3.4 × 10−7 | [33] |
| SiO2+Fe2O3 | TEOS+ Fe(NO3)3·9H2O | Sol–gel | 40 | 4.4 | 840 | −87 | 9.3 × 10−7 | [33] |
| SiO2+TiO2 | TEOS+TIP | CVD | 650 | 75 | 125 | −30 | 9.1 × 10−8 | [34] |
| SiO2+Nb2O5 | TEOS+NPB | Sol–gel | 200 | 56 | 70 | −32 | 2.6 × 10−8 | [35] |
| SiO2+Ta2O5 | TEOS+TaEO | CVD | 650 | 16 | 200 | −25 | 3.5 × 10−8 | [36] |
| Membrane ID | Ta/(Si+Ta) Fraction | TaEO Bubbler | Counter Flow Gas | Reactor Temperature | CVD Time /min | |
|---|---|---|---|---|---|---|
| Temperature | Flowrate | |||||
| /°C | /nmol s−1 | /°C | ||||
| Si-650 | - | - | - | Ar | 650 | 30 |
| Si-3Ta-650 | 0.03 | 117 | 0.29 | Ar | 650 | 30 |
| Si-10Ta-650 | 0.10 | 138 | 0.99 | Ar | 650 | 30 |
| Si-40Ta-650 | 0.40 | 173 | 6.1 | Ar | 650 | 30 |
| Si-10Ta-600 | 0.10 | 138 | 0.99 | Ar | 600 | 45 |
| Si-10Ta-500 | 0.10 | 138 | 0.99 | O2 | 500 | 30 |
| Si-10Ta-400 | 0.10 | 138 | 0.99 | O2 | 400 | 105 |
| XPS | EDS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Membrane ID | Elemental Fraction/% | Elemental Fraction/% | |||||||
| Before Sputtering | After Sputtering | ||||||||
| O1s | Al2p | Si2p | O1s | Al2p | Si2p | O | Al | Si | |
| Si-650 | 65 | 0.0 | 35 | 68 | 0.0 | 32 | 73 | 26 | 0.5 |
| Si-3Ta-650 | 68 | 0.0 | 32 | 67 | 1.5 | 32 | 81 | 14 | 4.2 |
| Si-10Ta-650 | 70 | 13 | 17 | 68 | 18 | 15 | 79 | 14 | 7.0 |
| Si-40Ta-650 | 66 | 4.6 | 30 | 65 | 10 | 24 | 80 | 13 | 6.6 |
| Si-10Ta-600 | 65 | 6.9 | 28 | 70 | 11 | 19 | 79 | 14 | 6.9 |
| Si-10Ta-500 | 70 | 7.4 | 23 | 66 | 16 | 18 | 79 | 15 | 6.3 |
| Si-10Ta-400 | 67 | 9.3 | 24 | 65 | 16 | 18 | 84 | 10 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundin, S.-T.B.; Wang, H.; Oyama, S.T. Synthesis and Characterization of Silica–Tantala Microporous Membranes for Gas Separations Fabricated Using Chemical Vapor Deposition. Membranes 2022, 12, 889. https://doi.org/10.3390/membranes12090889
Lundin S-TB, Wang H, Oyama ST. Synthesis and Characterization of Silica–Tantala Microporous Membranes for Gas Separations Fabricated Using Chemical Vapor Deposition. Membranes. 2022; 12(9):889. https://doi.org/10.3390/membranes12090889
Chicago/Turabian StyleLundin, Sean-Thomas B., Hongsheng Wang, and S. Ted Oyama. 2022. "Synthesis and Characterization of Silica–Tantala Microporous Membranes for Gas Separations Fabricated Using Chemical Vapor Deposition" Membranes 12, no. 9: 889. https://doi.org/10.3390/membranes12090889
APA StyleLundin, S. -T. B., Wang, H., & Oyama, S. T. (2022). Synthesis and Characterization of Silica–Tantala Microporous Membranes for Gas Separations Fabricated Using Chemical Vapor Deposition. Membranes, 12(9), 889. https://doi.org/10.3390/membranes12090889