Effect of Drying Temperature on Physical, Chemical, and Antioxidant Properties of Ginger Oil Loaded Gelatin-Sodium Alginate Edible Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
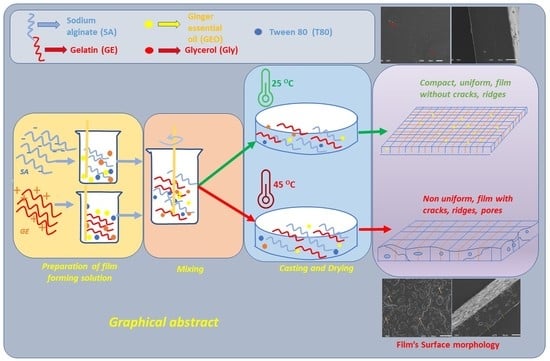
2.2. Preparation of the Films
2.3. SEM (Scanning Electron Microscopy) Analysis
2.4. Thermal Stability Assessment
2.5. Analysis of FTIR Spectra
2.6. X-ray Diffraction (XRD) Studies
2.7. Thickness
2.8. Mechanical Testing
2.9. Water Vapor Permeability (WVP)
2.10. Swelling Degree (SD) and Water Solubility (WS)
2.11. Moisture Content Analysis
2.12. Oxygen Barrier Property
2.13. Antioxidant Assays
2.13.1. Sample Preparation
2.13.2. Total Phenolic Content Assay
2.13.3. DPPH Assay
2.13.4. ABTS Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM)
3.2. Thermal Analysis
3.3. FTIR Data Analysis
3.4. X-ray Diffraction (XRD) Analysis
3.5. Film Thickness
3.6. Mechanical Properties
3.7. Water Vapor Permeability (WVP)
3.8. Oxygen Barrier Property (OB)
3.9. Swelling Degree (SD) and Water Solubility (WS)
3.10. Moisture Content (MC)
3.11. Optical Transmittance Analysis
3.12. Antioxidant Assays
3.12.1. DPPH Assay
3.12.2. ABTS Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Denavi, G.; Tapia-Blacido, D.R.; Añón, M.; Sobral, P.J.d.A.; Mauri, A.; Menegalli, F. Effects of drying conditions on some physical properties of soy protein films. J. Food Eng. 2009, 90, 341–349. [Google Scholar] [CrossRef]
- Liu, F.; Majeed, H.; Antoniou, J.; Li, Y.; Ma, Y.; Yokoyama, W.; Ma, J.; Zhong, F. Tailoring physical properties of transglutaminase-modified gelatin films by varying drying temperature. Food Hydrocoll. 2016, 58, 20–28. [Google Scholar] [CrossRef]
- Homez-Jara, A.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J. Hydrophilic edible films: Modified procedure for water vapor permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Kubra, I.R.; Rao, L.J.M. An impression on current developments in the technology, chemistry, and biological activities of ginger (Zingiber officinale Roscoe). Crit. Rev. Food Sci. Nutr. 2012, 52, 651–688. [Google Scholar] [CrossRef]
- ASTM D882-2010; Testing, AS f., & Materials: Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM International. Standard Test Method for Water Vapor Transmission of Materials. In Annual Book of ASTM Standard; ASTM International: West Conshohocken, PA, USA, 2000; Volume 14, pp. 878–885. [Google Scholar]
- Al-Harrasi, A.; Bhtaia, S.; Al-Azri, M.S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Mohan, S.; Sharma, A.; Behl, T. Development and characterization of chitosan and porphyran based composite edible films containing ginger essential oil. Polymers 2022, 14, 1782. [Google Scholar] [CrossRef]
- Romero-Bastida, C.A.; Bello-Pérez, L.A.; García, M.A.; Martino, M.N.; Solorza-Feria, J.; Zaritzky, N.E. Physicochemical and microstructural characterization of films prepared by thermal and cold gelatinization from non-conventional sources of starches. Carbohydr. Polym. 2005, 60, 235–244. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Dong, Z.; Zhang, T.; Yu, Q. Preparation and Characterization of Ginger Essential Oil Microcapsule Composite Films. Foods 2021, 10, 2268. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Golly, M.K.; Ma, H. Enhanced screening of key ultrasonication parameters: Total phenol content and antioxidant activity assessment of Tartary buckwheat (Fagopyrum tataricum) water extract. Sep. Sci. Technol. 2020, 55, 3242–3251. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Wang, J.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int. J. Biol. Macromol. 2019, 134, 1038–1044. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohydr. Polym. 2013, 98, 630–637. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W. Functional properties of gelatin from cuttlefish (Sepia pharaonis) skin as affected by bleaching using hydrogen peroxide. Food Chem. 2009, 115, 243–249. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of edible films based on nanoreinforced gelatinized starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Dou, B.; Dupont, V.; Williams, P.T.; Chen, H.; Ding, Y. Thermogravimetric kinetics of crude glycerol. Bioresour. Technol. 2009, 100, 2613–2620. [Google Scholar] [CrossRef] [Green Version]
- Apostolov, A.; Fakirov, S.; Vassileva, E.; Patil, R.; Mark, J. DSC and TGA studies of the behavior of water in native and crosslinked gelatin. J. Appl. Polym. Sci. 1999, 71, 465–470. [Google Scholar] [CrossRef]
- Qiao, D.; Wang, Z.; Cai, C.; Yin, S.; Qian, H.; Zhang, B.; Jiang, F.; Fei, X. Tailoring Multi-Level Structural and Practical Features of Gelatin Films by Varying Konjac Glucomannan Content and Drying Temperature. Polymers 2020, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.M.; Borges, S.V.; Marconcini, J.M.; Yoshida, M.I.; Neto, A.R.S.; Pereira, T.C.; Pereira, C.F.G. Effect of replacement of corn starch by whey protein isolate in biodegradable film blends obtained by extrusion. Carbohydr. Polym. 2017, 157, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolym. Orig. Res. Biomol. 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Cheng, Q.; Pan, F.; Jiang, Z. Sodium alginate–gelatin polyelectrolyte complex membranes with both high water vapor permeance and high permselectivity. J. Membr. Sci. 2011, 375, 304–312. [Google Scholar] [CrossRef]
- Cai, L.; Shi, H.; Cao, A.; Jia, J. Characterization of gelatin/chitosan ploymer films integrated with docosahexaenoic acids fabricated by different methods. Sci. Rep. 2019, 9, 8375. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, C.; Cho, S.; Kim, S.-B. Effects of thermal treatment on the physical properties of edible calcium alginate gel beads: Response surface methodological approach. Foods 2019, 8, 578. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Wang, K.; Wu, K.; Xiao, M.; Kuang, Y.; Corke, H.; Ni, X.; Jiang, F. Structural characterization and properties of konjac glucomannan and zein blend films. Int. J. Biol. Macromol. 2017, 105, 1096–1104. [Google Scholar] [CrossRef]
- Frank, K.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Alginate biocomposite films incorporated with cinnamon essential oil nanoemulsions: Physical, mechanical, and antibacterial properties. Int. J. Polym. Sci. 2018, 2018, 1519407. [Google Scholar] [CrossRef]
- Šuput, D.; Popović, S.; Hromiš, N.; Bulut, S.; Lazić, V. Biopolymer films properties change affected by essential oils addition. J. Process. Energy Agric. 2019, 23, 61–65. [Google Scholar] [CrossRef]
- Kalhoro, M.T.; Zhang, H.; Kalhoro, G.M.; Wang, F.; Chen, T.; Faqir, Y.; Nabi, F. Fungicidal properties of ginger (Zingiber officinale) essential oils against Phytophthora colocasiae. Sci. Rep. 2022, 12, 2191. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Sothornvit, R.; Pitak, N. Oxygen permeability and mechanical properties of banana films. Food Res. Int. 2007, 40, 365–370. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J. Oxygen and aroma barrier properties of edible films: A review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Avena-Bustillos, R.J.; Olsen, C.; Friedman, M.; Henika, P.R.; Martín-Belloso, O.; Pan, Z.; McHugh, T.H. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate–apple puree edible films. J. Food Eng. 2007, 81, 634–641. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Mestdagh, F.; De Meulenaer, B.; De Clippeleer, J.; Devlieghere, F.; Huyghebaert, A. Protective influence of several packaging materials on light oxidation of milk. J. Dairy Sci. 2005, 88, 499–510. [Google Scholar] [CrossRef]
- Xiu-Qin, L.; Chao, J.; Yan-Yan, S.; Min-Li, Y.; Xiao-Gang, C. Analysis of synthetic antioxidants and preservatives in edible vegetable oil by HPLC/TOF-MS. Food Chem. 2009, 113, 692–700. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Cava, R. Effect of natural and synthetic antioxidants on protein oxidation and colour and texture changes in refrigerated stored porcine liver pâté. Meat Sci. 2006, 74, 396–403. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Cao, J.; Zhao, S.; Wang, W. Microencapsulation of ginger volatile oil based on gelatin/sodium alginate polyelectrolyte complex. Chem. Pharm. Bull. 2016, 64, 21–26. [Google Scholar] [CrossRef] [Green Version]






| Composition | Drying Temperature | |
|---|---|---|
| 25 °C | 45 °C | |
| GE (3% w/v) + Gly (1.5% w/v) | F1 | F2 |
| GE (3% w/v) + Gly (1.5% w/v) + T80 (1%) + GEO (1%) | F3 | F4 |
| GE (3% w/v) + SA (3% w/v) + Gly (1.5% w/v) + T80 (1%) + GEO (1%) | F5 | F6 |
| Formulations | WVP (×10−12 G cm/cm2 s Pa) | OB (g/100 g) | Thickness (μm) | EB (%) | TS (MPa) | Ym (MPa) |
|---|---|---|---|---|---|---|
| F1 | 2.9 ± 0.02 c | 3.11 ± 0.013 a | 41.12 ± 1.2 c | 43.22 ± 8.14 d | 28.21 ± 1.36 e | 77.21 ± 1.67 b |
| F2 | 3.9 ± 0.01 a | 3.39 ± 0.043 a | 37.11 ± 2.4 d | 36.34 ± 6.11 e | 19.17 ± 27.1 f | 82.11 ± 3.11 a |
| F3 | 1.8 ± 0.02 d | 2.34 ± 0.059 b | 46.22 ± 1.9 b | 73.11 ± 4.26 b | 55.37 ± 4.28 b | 52.55 ± 6.18 d |
| F4 | 3.1 ± 0.03 b | 3.19 ± 0.049 a | 39.11± 2.3 d | 47.11 ± 7.21 d | 41.32 ± 1.24 d | 67.89. ± 2.81 c |
| F5 | 1.1 ± 0.02 d | 2.15 ± 0.031 b | 51.77 ± 1.8 a | 88.24 ± 7.14 a | 68.11 ± 3.24 a | 31.47 ± 2.14 f |
| F6 | 2.6 ± 0.01 c | 3.26 ± 0.047 a | 45.23 ± 1.1 b | 53.26± 4.11 c | 48.16 ± 6.12 c | 48.22 ± 3.18 e |
| Codes of the Samples | WS (%) | SD (%) | MC (%) | Transparency |
|---|---|---|---|---|
| F1 | 41.2 ± 1.2 a | 187 ± 15.1 a | 28.17 ± 0.14 a | 35.11 ± 1.25 b |
| F2 | 34.1 ± 0.6 b | 95 ± 17.6 c | 18.32 ± 0.21 c | 48.17 ± 2.15 a |
| F3 | 27.2 ± 0.3 c | 128 ± 11.4 b | 23.12 ± 0.25 b | 31.42 ± 1.42 c |
| F4 | 18.1 ± 3.2 d | 82 ± 6.1 d | 19.27 ± 0.16 c | 46.32 ± 2.11 a |
| F5 | 21.1 ± 1.2 e | 85 ± 8.1 d | 15.31 ± 0.23 d | 15.21 ± 0.31 e |
| F6 | 16.4 ± 0.1 d | 54 ± 2.8 e | 10.12 ± 0.37 e | 22.17 ± 0.11 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harrasi, A.; Bhatia, S.; Al-Azri, M.S.; Ullah, S.; Najmi, A.; Albratty, M.; Meraya, A.M.; Mohan, S.; Aldawsari, M.F. Effect of Drying Temperature on Physical, Chemical, and Antioxidant Properties of Ginger Oil Loaded Gelatin-Sodium Alginate Edible Films. Membranes 2022, 12, 862. https://doi.org/10.3390/membranes12090862
Al-Harrasi A, Bhatia S, Al-Azri MS, Ullah S, Najmi A, Albratty M, Meraya AM, Mohan S, Aldawsari MF. Effect of Drying Temperature on Physical, Chemical, and Antioxidant Properties of Ginger Oil Loaded Gelatin-Sodium Alginate Edible Films. Membranes. 2022; 12(9):862. https://doi.org/10.3390/membranes12090862
Chicago/Turabian StyleAl-Harrasi, Ahmed, Saurabh Bhatia, Mohammed Said Al-Azri, Sana Ullah, Asim Najmi, Mohammed Albratty, Abdulkarim M. Meraya, Syam Mohan, and Mohammed F. Aldawsari. 2022. "Effect of Drying Temperature on Physical, Chemical, and Antioxidant Properties of Ginger Oil Loaded Gelatin-Sodium Alginate Edible Films" Membranes 12, no. 9: 862. https://doi.org/10.3390/membranes12090862
APA StyleAl-Harrasi, A., Bhatia, S., Al-Azri, M. S., Ullah, S., Najmi, A., Albratty, M., Meraya, A. M., Mohan, S., & Aldawsari, M. F. (2022). Effect of Drying Temperature on Physical, Chemical, and Antioxidant Properties of Ginger Oil Loaded Gelatin-Sodium Alginate Edible Films. Membranes, 12(9), 862. https://doi.org/10.3390/membranes12090862