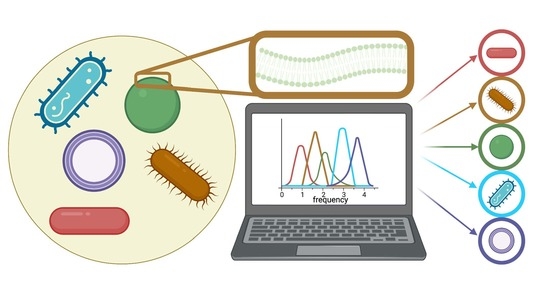
Low-THz Vibrations of Biological Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systems
2.2. Molecular Dynamics Simulations
2.3. Spectra
2.4. Signal Analysis
2.5. Root-Mean-Squared Fluctuations (RMSF)
3. Results
3.1. Membrane Asymmetry
3.2. Cell Type
3.3. Sterols
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | cardiolipin |
| DAG | diacylglycerol |
| FA | free fatty acid |
| KS | Kolmogorov-Smirnov |
| LPG | lysophosphatidylglycerol |
| MD | Molecular Dynamics |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
| PSM | sphingomyelin |
| S476 | Asymmetric S. aureus membrane with 33% LPG content |
| S476 | Symmetric S. aureus membrane with 33% LPG content |
| S476 | Asymmetric S. aureus membrane with 51% LPG content |
References
- Leonov, D.V.; Dzuba, S.A.; Surovtsev, N.V. Normal vibrations of ternary DOPC/DPPC/cholesterol lipid bilayers by low-frequency Raman spectroscopy. RSC Adv. 2019, 9, 34451–34456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Elvati, P.; Majumder, S.; Wang, Y.; Liu, A.P.; Violi, A. Predicting the Time of Entry of Nanoparticles in Lipid Membranes. ACS Nano 2019, 13, 10221–10232. [Google Scholar] [CrossRef] [PubMed]
- Paula, S.; Volkov, A.; Van Hoek, A.; Haines, T.; Deamer, D.W. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 1996, 70, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhernenkov, M.; Bolmatov, D.; Soloviov, D.; Zhernenkov, K.; Toperverg, B.P.; Cunsolo, A.; Bosak, A.; Cai, Y.Q. Revealing the mechanism of passive transport in lipid bilayers via phonon-mediated nanometre-scale density fluctuations. Nat. Commun. 2016, 7, 12292. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liao, C.; Huang, H.; Weiss, T.; Bellisent-Funel, M.; Sette, F. Collective dynamics in fully hydrated phospholipid bilayers studied by inelastic X-ray scattering. Phys. Rev. Lett. 2001, 86, 740. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Elvati, P.; Violi, A. On Drug-Membrane Permeability of Antivirals for SARS-CoV-2. J. Phys. Chem. Lett. 2021, 12, 1384–1389. [Google Scholar] [CrossRef]
- Rheinstädter, M.; Ollinger, C.; Fragneto, G.; Demmel, F.; Salditt, T. Collective dynamics of lipid membranes studied by inelastic neutron scattering. Phys. Rev. Lett. 2004, 93, 108107. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, C.L.; Barrett, M.A.; Hiess, A.; Salditt, T.; Katsaras, J.; Shi, A.C.; Rheinstädter, M.C. Effect of cholesterol on the lateral nanoscale dynamics of fluid membranes. Eur. Biophys. J. 2012, 41, 901–913. [Google Scholar] [CrossRef]
- Surovtsev, N.; Adichtchev, S. Dynamic response on a nanometer scale of binary phospholipid-cholesterol vesicles: Low-frequency Raman scattering insight. Phys. Rev. E 2021, 104, 054406. [Google Scholar] [CrossRef]
- Foley, S.L.; Hossein, A.; Deserno, M. Fluid-gel coexistence in lipid membranes under differential stress. Biophys. J. 2022, 121, 2997–3009. [Google Scholar] [CrossRef]
- Rosado, H.; Turner, R.D.; Foster, S.J.; Taylor, P.W. Impact of the β-Lactam resistance modifier (-)-epicatechin gallate on the non-random distribution of phospholipids across the cytoplasmic membrane of staphylococcus aureus. Int. J. Mol. Sci. 2015, 16, 16710–16727. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.; Yeaman, M.R.; Sakoulas, G.; Yang, S.J.; Proctor, R.A.; Sahl, H.G.; Schrenzel, J.; Xiong, Y.Q.; Bayer, A.S. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 2008, 52, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shireen, T.; Singh, M.; Das, T.; Mukhopadhyay, K. Differential adaptive responses of Staphylococcus aureus to in vitro selection with different antimicrobial peptides. Antimicrob. Agents Chemother. 2013, 57, 5134–5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coblentz, W.W. Radiometric Investigation of Water of Crystallization, Light Filters, and Standard Absorption Bands; Number 168; US Government Printing Office: Washington, DC, USA, 1911. [Google Scholar]
- Horbach, I.; Naumann, D.; Fehrenbach, F.J. Simultaneous infections with different serogroups of Legionella pneumophila investigated by routine methods and Fourier transform infrared spectroscopy. J. Clin. Microbiol. 1988, 26, 1106–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, D.; Helm, D.; Labischinski, H. Microbiological characterizations by FT-IR spectroscopy. Nature 1991, 351, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Curk, M.; Peledan, F.; Hubert, J. Fourier transform infrared (FTIR) spectroscopy for identifying Lactobacillus species. FEMS Microbiol. Lett. 1994, 123, 241–248. [Google Scholar] [CrossRef]
- Helm, D.; Labischinski, H.; Schallehn, G.; Naumann, D. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. Microbiology 1991, 137, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, D.; Fijala, V.; Labischinski, H.; Giesbrecht, P. The rapid differentiation and identification of pathogenic bacteria using Fourier transform infrared spectroscopic and multivariate statistical analysis. J. Mol. Struct. 1988, 174, 165–170. [Google Scholar] [CrossRef]
- Thomas, L.; Greenstreet, J. The identification of micro-organisms by infrared spectrophotometry. Spectrochim. Acta 1954, 6, 302–319. [Google Scholar] [CrossRef]
- Kenner, B.A.; Riddle, J.W.; Rockwood, S.W.; Bordner, R.H. Bacterial Identification by Infrared Spectrophotometry II: Effect of Instrumental and Environmental Variables. J. Bacteriol. 1958, 75, 16–20. [Google Scholar] [CrossRef]
- Berrier, A.; Schaafsma, M.C.; Nonglaton, G.; Bergquist, J.; Rivas, J.G. Selective detection of bacterial layers with terahertz plasmonic antennas. Biomed. Opt. Express 2012, 3, 2937–2949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Cha, S.; Jun, S.; Park, S.; Park, J.Y.; Lee, S.; Kim, H.; Ahn, Y. Identifying different types of microorganisms with terahertz spectroscopy. Biomed. Opt. Express 2020, 11, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Barani, N.; Sarabandi, K. Electromagnetic Signaling and Quorum Sensing within Biofilms: Which Mechanism Is the Most Probable Means of Communication? In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; IEEE: New York, NY, USA, 2020; pp. 2459–2462. [Google Scholar]
- Barani, N.; Sarabandi, K.; Kotov, N.A.; Vanepps, J.S.; Elvati, P.; Wang, Y.; Violi, A. A Multiphysics Modeling of Electromagnetic Signaling Phenomena at kHz-GHz Frequencies in Bacterial Biofilms. IEEE Access 2022, 10, 39344–39361. [Google Scholar] [CrossRef]
- Barani, N.; Kashanianfard, M.; Sarabandi, K. A Mechanical Antenna with Frequency Multiplication and Phase Modulation Capability. IEEE Trans. Antennas Propag. 2020, 69, 3726–3739. [Google Scholar] [CrossRef]
- Montagnier, L.; Aissa, J.; Ferris, S.; Montagnier, J.L.; Lavalléee, C. Electromagnetic signals are produced by aqueous nanostructures derived from bacterial DNA sequences. Interdiscip. Sci. Comput. Life Sci. 2009, 1, 81–90. [Google Scholar] [CrossRef]
- Turton, D.A.; Senn, H.M.; Harwood, T.; Lapthorn, A.J.; Ellis, E.M.; Wynne, K. Terahertz underdamped vibrational motion governs protein-ligand binding in solution. Nat. Commun. 2014, 5, 3999. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Plaxco, K.W.; Allen, S.J. Probing the collective vibrational dynamics of a protein in liquid water by terahertz absorption spectroscopy. Protein Sci. 2006, 15, 1175–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.J.; Cheng, G.; Huang, Z.; Zhang, S.; Norris, T.B.; Kotov, N.A. Terahertz circular dichroism spectroscopy of biomaterials enabled by kirigami polarization modulators. Nat. Mater. 2019, 18, 820–826. [Google Scholar] [CrossRef]
- Piggot, T.J.; Holdbrook, D.A.; Khalid, S. Electroporation of the E. coli and S. aureus membranes: Molecular dynamics simulations of complex bacterial membranes. J. Phys. Chem. B 2011, 115, 13381–13388. [Google Scholar] [CrossRef]
- Brandt, E.G.; Braun, A.R.; Sachs, J.N.; Nagle, J.F.; Edholm, O. Interpretation of fluctuation spectra in lipid bilayer simulations. Biophys. J. 2011, 100, 2104–2111. [Google Scholar] [CrossRef]
- Braun, A.R.; Brandt, E.G.; Edholm, O.; Nagle, J.F.; Sachs, J.N. Determination of electron density profiles and area from simulations of undulating membranes. Biophys. J. 2011, 100, 2112–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boek, E.; Padding, J.; den Otter, W.K.; Briels, W.J. Mechanical properties of surfactant bilayer membranes from atomistic and coarse-grained molecular dynamics simulations. J. Phys. Chem. B 2005, 109, 19851–19858. [Google Scholar] [CrossRef] [PubMed]
- Stecki, J. Variation of lateral tension and a new transition in model bilayers made of chain molecules. J. Chem. Phys. 2005, 122, 111102. [Google Scholar] [CrossRef]
- Stecki, J. Note: On the power spectrum of undulations of simulated bilayers. J. Chem. Phys. 2012, 137, 116102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stecki, J. Correlations in simulated model bilayers. J. Chem. Phys. 2004, 120, 3508–3516. [Google Scholar] [CrossRef]
- Różycki, B.; Lipowsky, R. Spontaneous curvature of bilayer membranes from molecular simulations: Asymmetric lipid densities and asymmetric adsorption. J. Chem. Phys. 2015, 142, 02B601_1. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; He, Z.; Yu, W. Comparison of public peak detection algorithms for MALDI mass spectrometry data analysis. BMC Bioinform. 2009, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lipkus, A.H.; Chittur, K.K.; Vesper, S.J.; Robinson, J.B.; Pierce, G.E. Evaluation of infrared spectroscopy as a bacterial identification method. J. Ind. Microbiol. 1990, 6, 71–75. [Google Scholar] [CrossRef]
- Helm, D.; Labischinski, H.; Naumann, D. Elaboration of a procedure for identification of bacteria using Fourier-transform IR spectral libraries: A stepwise correlation approach. J. Microbiol. Methods 1991, 14, 127–142. [Google Scholar] [CrossRef]
- Naumann, D. The ultra rapid differentiation and identification of pathogenic bacteria using FT-IR techniques. In Fourier and computerized Infrared Spectroscopy; SPIE: Bellingham, WA, USA, 1985; Volume 553, pp. 268–269. [Google Scholar]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [Green Version]
- Kourtis, A.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.; Dumyati, G.; Petit, S.; et al. Emerging Infections Program MRSA Author Group: Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections-United States. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.J.; Haste, N.M.; Hollands, A.; Fleming, T.C.; Hamby, M.; Pogliano, K.; Nizet, V.; Dorrestein, P.C. Microbial competition between Bacillus subtilis and Staphylococcus aureus monitored by imaging mass spectrometry. Microbiology 2011, 157, 2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehal, R.P.; Marbach, H.; Hubbard, A.T.; Sacranie, A.A.; Sebastiani, F.; Fragneto, G.; Harvey, R.D. The influence of mild acidity on lysyl-phosphatidylglycerol biosynthesis and lipid membrane physico-chemical properties in methicillin-resistant Staphylococcus aureus. Chem. Phys. Lipids 2017, 206, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Drew Bennett, W.; Fox, S.J.; Sun, D.; Maupin, C.M. Bacterial Membranes Are More Perturbed by the Asymmetric Versus Symmetric Loading of Amphiphilic Molecules. Membranes 2022, 12, 350. [Google Scholar] [CrossRef]
- White, D.C.; Frerman, F.E. Fatty acid composition of the complex lipids of Staphylococcus aureus during the formation of the membrane-bound electron transport system. J. Bacteriol. 1968, 95, 2198–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haest, C.; De Gier, J.; Den Kamp, J.O.; Bartels, P.; Van Deenen, L. Changes in permeability of Staphylococcus aureus and derived liposomes with varying lipid composition. Biochim. Biophys. Acta BBA-Biomembr. 1972, 255, 720–733. [Google Scholar] [CrossRef] [Green Version]
- Gould, R.M.; Lennarz, W. Metabolism of phosphatidylglycerol and lysyl phosphatidylglycerol in Staphylococcus aureus. J. Bacteriol. 1970, 104, 1135–1144. [Google Scholar] [CrossRef] [Green Version]
- Witzke, S.; Petersen, M.; Carpenter, T.S.; Khalid, S. Molecular dynamics simulations reveal the conformational flexibility of lipid II and its loose association with the defensin plectasin in the Staphylococcus aureus membrane. Biochemistry 2016, 55, 3303–3314. [Google Scholar] [CrossRef] [Green Version]
- Hayami, M.; Okabe, A.; Kariyama, R.; Abe, M.; Kanemasa, Y. Lipid composition of Staphylococcus aureus and its derived L-forms. Microbiol. Immunol. 1979, 23, 435–442. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327.61. [Google Scholar] [CrossRef] [Green Version]
- Gowers, R.J.; Linke, M.; Barnoud, J.; Reddy, T.J.; Melo, M.N.; Seyler, S.L.; Domanski, J.; Dotson, D.L.; Buchoux, S.; Kenney, I.M.; et al. MDAnalysis: A Python package for the rapid analysis of molecular dynamics simulations. In Proceedings of the 15th Python in Science Conference (SciPy), Austin, TX, USA, 11–17 July 2016; Volume 98, p. 105. [Google Scholar]
- Ramírez, R.; López-Ciudad, T.; Kumar, P.P.; Marx, D. Quantum corrections to classical time-correlation functions: Hydrogen bonding and anharmonic floppy modes. J. Chem. Phys. 2004, 121, 3973–3983. [Google Scholar] [CrossRef]
- Wilmink, G.J.; Ibey, B.L.; Rivest, B.D.; Grundt, J.E.; Roach, W.P.; Tongue, T.D.; Schulkin, B.J.; Laman, N.; Peralta, X.G.; Roth, C.C.; et al. Development of a compact terahertz time-domain spectrometer for the measurement of the optical properties of biological tissues. J. Biomed. Opt. 2011, 16, 047006. [Google Scholar] [CrossRef]
- Hodges, J.L. The significance probability of the Smirnov two-sample test. Ark. Mat. 1958, 3, 469–486. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Wang, L.; Jing, T.; Chen, J.; Xu, X.; Zhang, W.; Zhang, Y.; Han, J. Unusual features and molecular pathways of Staphylococcus aureus L-form bacteria. Microb. Pathog. 2020, 140, 103970. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamaguchi, H. Morphological Detection of Filipin-Sterol Complexes in the Cytoplasmic Membrane of Staphylococcal L-Form. Microbiol. Immunol. 1990, 34, 25–34. [Google Scholar] [CrossRef]
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Kilelee, E.; Pokorny, A.; Yeaman, M.R.; Bayer, A.S. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic antimicrobial peptide 6W-RP-1 in a model membrane system: Implications for daptomycin resistance. Antimicrob. Agents Chemother. 2010, 54, 4476–4479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wepy, J.A.; Galligan, J.J.; Kingsley, P.J.; Xu, S.; Goodman, M.C.; Tallman, K.A.; Rouzer, C.A.; Marnett, L.J. Lysophospholipases cooperate to mediate lipid homeostasis and lysophospholipid signaling [S]. J. Lipid Res. 2019, 60, 360–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, W.J.; Chambers, K.; Doody, A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic 2003, 4, 214–221. [Google Scholar] [CrossRef]
- Grzelczyk, A.; Gendaszewska-Darmach, E. Novel bioactive glycerol-based lysophospholipids: New data–new insight into their function. Biochimie 2013, 95, 667–679. [Google Scholar] [CrossRef]
- Xu, W.; Hsu, F.F.; Baykal, E.; Huang, J.; Zhang, K. Sterol biosynthesis is required for heat resistance but not extracellular survival in Leishmania. PLoS Pathog. 2014, 10, e1004427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, A.T.; Barker, R.; Rehal, R.; Vandera, K.K.A.; Harvey, R.D.; Coates, A.R. Mechanism of Action of a Membrane-Active Quinoline-Based Antimicrobial on Natural and Model Bacterial Membranes. Biochemistry 2017, 56, 1163–1174. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luyet, C.; Elvati, P.; Vinh, J.; Violi, A. Low-THz Vibrations of Biological Membranes. Membranes 2023, 13, 139. https://doi.org/10.3390/membranes13020139
Luyet C, Elvati P, Vinh J, Violi A. Low-THz Vibrations of Biological Membranes. Membranes. 2023; 13(2):139. https://doi.org/10.3390/membranes13020139
Chicago/Turabian StyleLuyet, Chloe, Paolo Elvati, Jordan Vinh, and Angela Violi. 2023. "Low-THz Vibrations of Biological Membranes" Membranes 13, no. 2: 139. https://doi.org/10.3390/membranes13020139
APA StyleLuyet, C., Elvati, P., Vinh, J., & Violi, A. (2023). Low-THz Vibrations of Biological Membranes. Membranes, 13(2), 139. https://doi.org/10.3390/membranes13020139