Phospholipases and Membrane Curvature: What Is Happening at the Surface?
Abstract
:1. Introduction
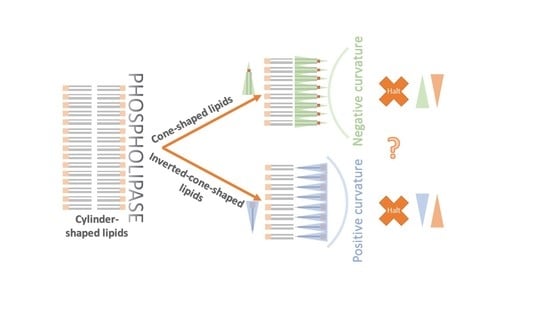
2. The Basics: Molecular Shapes and Membrane Curvature
3. On Membrane Curvature and Signal Transduction
4. Membrane Curvature and the Recruitment of Protein Factors
5. Membrane Fusion and Cell Function
6. Pharmacologic Modulation of Membrane Curvature
7. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verstraeten, S.V.; Mackenzie, G.G.; Oteiza, P.I. The Plasma Membrane Plays a Central Role in Cells Response to Mechanical Stress. Biochim. Biophys. Acta 2010, 1798, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Fanani, M.L.; Hartel, S.; Maggio, B.; De Tullio, L.; Jara, J.; Olmos, F.; Oliveira, R.G. The Action of Sphingomyelinase in Lipid Monolayers as Revealed by Microscopic Image Analysis. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Gudmand, M.; Rocha, S.; Hatzakis, N.S.; Peneva, K.; Müllen, K.; Stamou, D.; Uji-I, H.; Hofkens, J.; Bjørnholm, T.; Heimburg, T. Influence of Lipid Heterogeneity and Phase Behavior on Phospholipase A 2 Action at the Single Molecule Level. Biophys. J. 2010, 98, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; De Keersmaecker, H.; Hutchison, J.A.; Vanhoorelbeke, K.; Martens, J.A.; Hofkens, J.; Uji-I, H. Membrane Remodeling Processes Induced by Phospholipase Action. Langmuir 2014, 30, 4743–4751. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A. Membrane Fusion Induced by Phospholipase C and Sphingomyelinases. Biosci. Rep. 2000, 20, 443–463. [Google Scholar] [CrossRef]
- Maggio, B.; Borioli, G.a.; Boca, M.; Tullio, L.; Fanani, M.L.; Oliveira, R.G.; Rosetti, C.M.; Wilke, N. Composition-Driven Surface Domain Structuring Mediated by Sphingolipids and Membrane-Active Proteins. Cell Biochem. Biophys. 2008, 50, 79–109. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Andresen, T.L.; Halperin, A.; Hansen, P.L.; Jakobsen, A.F.; Jensen, U.B.; Jensen, M.O.; Jørgensen, K.; Kaasgaard, T.; Leidy, C.; et al. Activation of Interfacial Enzymes at Membrane Surfaces. J. Phys. Condens. Matter 2006, 18, S1293–S1304. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Fanani, M.L.; De Tullio, L.; Hartel, S.; Jara, J.; Maggio, B. Sphingomyelinase-Induced Domain Shape Relaxation Driven by Out-of-Equilibrium Changes of Composition. Biophys. J. 2009, 96, 67–76. [Google Scholar] [CrossRef]
- Chiou, Y.L.; Lin, S.R.; Chang, L. Sen Lipid Domain Formation Modulates Activities of Snake Venom Phospholipase A2 Enzymes. Toxicon 2010, 56, 1362–1371. [Google Scholar] [CrossRef]
- Silva, L.C.; Futerman, A.H.; Prieto, M. Lipid Raft Composition Modulates Sphingomyelinase Activity and Ceramide-Induced Membrane Physical Alterations. Biophys. J. 2009, 96, 3210–3222. [Google Scholar] [CrossRef]
- Fanani, M.L.; Härtel, S.; Oliveira, R.G.; Maggio, B. Bidirectional Control of Sphingomyelinase Activity and Surface Topography in Lipid Monolayers. Biophys. J. 2002, 83, 3416–3424. [Google Scholar] [CrossRef] [PubMed]
- Leidy, C.; Ocampo, J.; Duelund, L.; Mouritsen, O.G.; Jørgensen, K.; Peters, G.H. Membrane Restructuring by Phospholipase A 2 Is Regulated by the Presence of Lipid Domains. Biophys. J. 2011, 101, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Goñi, F.M.; Montes, L.-R.; Alonso, A. Phospholipases C and Sphingomyelinases: Lipids as Substrates and Modulators of Enzyme Activity. Prog. Lipid Res. 2012, 51, 238–266. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.M.; Angelova, M.I.; Kinnunen, P.K. Vectorial Budding of Vesicles by Asymmetrical Enzymatic Formation of Ceramide in Giant Liposomes. Biophys. J. 2000, 78, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Staneva, G.; Angelova, M.I.; Koumanov, K. Phospholipase A2 Promotes Raft Budding and Fission from Giant Liposomes. Chem. Phys. Lipids 2004, 129, 53–62. [Google Scholar] [CrossRef]
- Montes, L.R.; Ruiz-Argüello, M.B.; Goñi, F.M.; Alonso, A. Membrane Restructuring via Ceramide Results in Enhanced Solute Efflux. J. Biol. Chem. 2002, 277, 11788–11794. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press: London, UK, 1991; ISBN 0-12-375181-0. [Google Scholar]
- Tanford, C. Hydrophobic Free Energy, Micelle Formation and the Association of Proteins with Amphiphiles. J. Mol. Biol. 1972, 61, 59–74. [Google Scholar] [CrossRef]
- Kobierski, J.; Wnętrzak, A.; Chachaj-Brekiesz, A.; Dynarowicz-Latka, P. Predicting the Packing Parameter for Lipids in Monolayers with the Use of Molecular Dynamics. Colloids Surfaces B Biointerfaces 2022, 211, 112298. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Bagatolli, L.A. Life—As a Matter of Fat, 2nd ed.; The Frontiers Collection; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-22613-2. [Google Scholar]
- Kumar, V.V. Complementary Molecular Shapes and Additivity of the Packing Parameter of Lipids. Proc. Natl. Acad. Sci. USA 1991, 88, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A Simple Sphingolipid with Unique Biophysical Properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Fanani, M.L.; Maggio, B. The Many Faces (and Phases) of Ceramide and Sphingomyelin I—Single Lipids. Biophys. Rev. 2017, 9, 589–600. [Google Scholar] [CrossRef]
- Galassi, V.V.; Wilke, N. On the Coupling between Mechanical Properties and Electrostatics in Biological Membranes. Membranes 2021, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.R.; Kassas, N.; Chasserot-Golaz, S.; Bader, M.F.; Vitale, N. Lipids in Regulated Exocytosis: What Are They Doing? Front. Endocrinol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Peñalva, D.A.; Antollini, S.S.; Ambroggio, E.E.; Aveldaño, M.I.; Fanani, M.L. Membrane Restructuring Events during the Enzymatic Generation of Ceramides with Very Long-Chain Polyunsaturated Fatty Acids. Langmuir 2018, 34, 4398–4407. [Google Scholar] [CrossRef]
- Brown, W.J.; Chambers, K.; Doody, A. Phospholipase A2 (PLA2) Enzymes in Membrane Trafficking: Mediators of Membrane Shape and Function. Traffic 2003, 4, 214–221. [Google Scholar] [CrossRef]
- Ibarguren, M.; Bomans, P.H.H.; Frederik, P.M.; Stonehouse, M.; Vasil, A.I.; Vasil, M.L.; Alonso, A.; Goñi, F.M. End-Products Diacylglycerol and Ceramide Modulate Membrane Fusion Induced by a Phospholipase C/Sphingomyelinase from Pseudomonas Aeruginosa. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 59–64. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Jennings, W.; Black, S.; Hou, Y.H.; Lameire, D.; Chatha, P.; Kimura, T.; Berno, B.; Khondker, A.; Rheinstädter, M.C.; et al. Membrane Curvature Allosterically Regulates the Phosphatidylinositol Cycle, Controlling Its Rate and Acyl-Chain Composition of Its Lipid Intermediates. J. Biol. Chem. 2018, 293, 17780–17791. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Epand, R.M. DGKα, Bridging Membrane Shape Changes with Specific Molecular Species of DAG/PA: Implications in Cancer and Immunosurveillance. Cancers 2022, 14, 5259. [Google Scholar] [CrossRef]
- Schmick, M.; Bastiaens, P.I.H. The Interdependence of Membrane Shape and Cellular Signal Processing. Cell 2014, 156, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Grebenkov, D.S.; Metzler, R.; Oshanin, G. Search Efficiency in the Adam-Delbrück Reduction-of-Dimensionality Scenario versus Direct Diffusive Search. New J. Phys. 2022, 24, 083035. [Google Scholar] [CrossRef]
- Daniels, D.R. Receptor-Ligand Diffusion-Limited Reaction Rates on Curved Membranes. Chem. Phys. Lett. 2022, 795, 139516. [Google Scholar] [CrossRef]
- Davies, S.M.A.; Epand, R.M.; Kraayenhof, R.; Cornell, R.B. Regulation of CTP: Phosphocholine Cytidylyltransferase Activity by the Physical Properties of Lipid Membranes: An Important Role for Stored Curvature Strain Energy. Biochemistry 2001, 40, 10522–10531. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.B.; Arnold, R.S. Modulation of the Activities of Enzymes of Membrane Lipid Metabolism by Non-Bilayer-Forming Lipids. Chem. Phys. Lipids 1996, 81, 215–227. [Google Scholar] [CrossRef]
- Drobnies, A.E.; Davies, S.M.; Kraayenhof, R.; Epand, R.F.; Epand, R.M.; Cornell, R.B. CTP:Phosphocholine Cytidylyltransferase and Protein Kinase C Recognize Different Physical Features of Membranes: Differential Responses to an Oxidized Phosphatidylcholine. Biochim. Biophys. Acta-Biomembr. 2002, 1564, 82–90. [Google Scholar] [CrossRef]
- Attard, G.S.; Templer, R.H.; Smith, W.S.; Hunt, A.N.; Jackowski, S. Modulation of CTP:Phosphocholine Cytidylyltransferase by Membrane Curvature Elastic Stress. Proc. Natl. Acad. Sci. USA 2000, 97, 9032–9036. [Google Scholar] [CrossRef]
- Haider, A.; Wei, Y.C.; Lim, K.; Barbosa, A.D.; Liu, C.H.; Weber, U.; Mlodzik, M.; Oras, K.; Collier, S.; Hussain, M.M.; et al. PCYT1A Regulates Phosphatidylcholine Homeostasis from the Inner Nuclear Membrane in Response to Membrane Stored Curvature Elastic Stress. Dev. Cell 2018, 45, 481–495.e8. [Google Scholar] [CrossRef] [PubMed]
- Bahja, J.; Dymond, M.K. Does Membrane Curvature Elastic Energy Play a Role in Mediating Oxidative Stress in Lipid Membranes? Free Radic. Biol. Med. 2021, 171, 191–202. [Google Scholar] [CrossRef]
- Zemel, A.; Ben-Shaul, A.; May, S. Modulation of the Spontaneous Curvature and Bending Rigidity of Lipid Membranes by Interfacially Adsorbed Amphipathic Peptides. J. Phys. Chem. B 2008, 112, 6988–6996. [Google Scholar] [CrossRef]
- Seddon, J.M.; Templer, R.H. Polymorphism of Lipid-Water Systems. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1995; Volume 1, pp. 97–160. ISBN 0-444-81975-4. [Google Scholar]
- Has, C.; Das, S.L. Recent Developments in Membrane Curvature Sensing and Induction by Proteins. Biochim. Biophys. Acta-Gen. Subj. 2021, 1865, 129971. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, J.C.; Schmid, E.M.; Ryan, C.J.; Ann, H.S.; Sasaki, D.Y.; Sherman, M.B.; Geissler, P.L.; Fletcher, D.A.; Hayden, C.C. Membrane Bending by Protein–Protein Crowding. Nat. Cell Biol. 2012, 14, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Yunes Quartino, P.; Fidelio, G.D.; Manneville, J.-B.; Goud, B.; Ambroggio, E.E. Detecting Phospholipase Activity with the Amphipathic Lipid Packing Sensor Motif of ArfGAP1. Biochem. Biophys. Res. Commun. 2018, 505, 290–294. [Google Scholar] [CrossRef]
- Ambroggio, E.; Sorre, B.; Bassereau, P.; Goud, B.; Manneville, J.-B.; Antonny, B. ArfGAP1 Generates an Arf1 Gradient on Continuous Lipid Membranes Displaying Flat and Curved Regions. EMBO J. 2010, 29, 292–303. [Google Scholar] [CrossRef]
- Melero, A.; Chiaruttini, N.; Karashima, T.; Riezman, I.; Funato, K.; Barlowe, C.; Riezman, H.; Roux, A. Lysophospholipids Facilitate COPII Vesicle Formation. Curr. Biol. 2018, 28, 1950–1958.e6. [Google Scholar] [CrossRef] [PubMed]
- Anitei, M.; Stange, C.; Czupalla, C.; Niehage, C.; Schuhmann, K.; Sala, P.; Czogalla, A.; Pursche, T.; Coskun, Ü.; Shevchenko, A.; et al. Spatiotemporal Control of Lipid Conversion, Actin-Based Mechanical Forces, and Curvature Sensors during Clathrin/AP-1-Coated Vesicle Biogenesis. Cell Rep. 2017, 20, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Ibarguren, M.; Bomans, P.H.H.; Ruiz-Mirazo, K.; Frederik, P.M.; Alonso, A.; Goñi, F.M. Thermally-Induced Aggregation and Fusion of Protein-Free Lipid Vesicles. Colloids Surfaces B Biointerfaces 2015, 136, 545–552. [Google Scholar] [CrossRef]
- Lira, R.B.; Dimova, R. Fusion Assays for Model Membranes: A Critical Review. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 229–270. [Google Scholar]
- Heimburg, T. Thermal Biophysics of Membranes; WILEY-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2007; ISBN 9783527404711. [Google Scholar]
- Marrink, S.J.; Mark, A.E. The Mechanism of Vesicle Fusion as Revealed by Molecular Dynamics Simulations. J. Am. Chem. Soc. 2003, 125, 11144–11145. [Google Scholar] [CrossRef]
- Meher, G.; Chakraborty, H. Membrane Composition Modulates Fusion by Altering Membrane Properties and Fusion Peptide Structure. J. Membr. Biol. 2019, 252, 261–272. [Google Scholar] [CrossRef]
- Mondal Roy, S.; Sarkar, M. Membrane Fusion Induced by Small Molecules and Ions. J. Lipids 2011, 2011, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lira, R.B.; Robinson, T.; Dimova, R.; Riske, K.A. Highly Efficient Protein-Free Membrane Fusion: A Giant Vesicle Study. Biophys. J. 2019, 116, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.N.; Laviad, E.L.; Stiban, J.; Kelly, S.L.; Merrill, A.H.; Prieto, M.; Futerman, A.H.; Silva, L.C. Changes in Membrane Biophysical Properties Induced by Sphingomyelinase Depend on the Sphingolipid N-Acyl Chain. J. Lipid Res. 2014, 55, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Palfreyman, M.T.; Jorgensen, E.M. Roles of SNARE Proteins in Synaptic Vesicle Fusion. In Molecular Mechanisms of Neurotransmitter Release; Humana Press: Totowa, NJ, USA, 2008; pp. 35–59. ISBN 1597454818. [Google Scholar]
- Dabral, D.; Coorssen, J.R. Phospholipase A2: Potential Roles in Native Membrane Fusion. Int. J. Biochem. Cell Biol. 2017, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dabral, D.; Coorssen, J.R. Arachidonic Acid and Lysophosphatidylcholine Inhibit Multiple Late Steps of Regulated Exocytosis. Biochem. Biophys. Res. Commun. 2019, 515, 261–267. [Google Scholar] [CrossRef]
- Villanueva, J.; Gimenez-Molina, Y.; Davletov, B.; Gutiérrez, L.M. Vesicle Fusion as a Target Process for the Action of Sphingosine and Its Derived Drugs. Int. J. Mol. Sci. 2022, 23, 1086. [Google Scholar] [CrossRef]
- Parrish, C.R. Structures and Functions of Parvovirus Capsids and the Process of Cell Infection. In Cell Entry by Non-Enveloped Viruses; Springer: Berlin/Heidelberg, Germany, 2010; pp. 149–176. [Google Scholar]
- Bleker, S.; Sonntag, F.; Kleinschmidt, J.A. Mutational Analysis of Narrow Pores at the Fivefold Symmetry Axes of Adeno-Associated Virus Type 2 Capsids Reveals a Dual Role in Genome Packaging and Activation of Phospholipase A2 Activity. J. Virol. 2005, 79, 2528–2540. [Google Scholar] [CrossRef]
- Grieger, J.C.; Johnson, J.S.; Gurda-Whitaker, B.; Agbandje-McKenna, M.; Samulski, R.J. Surface-Exposed Adeno-Associated Virus Vp1-NLS Capsid Fusion Protein Rescues Infectivity of Noninfectious Wild-Type Vp2/Vp3 and Vp3-Only Capsids but Not That of Fivefold Pore Mutant Virions. J. Virol. 2007, 81, 7833–7843. [Google Scholar] [CrossRef]
- Parker, J.S.L.; Parrish, C.R. Cellular Uptake and Infection by Canine Parvovirus Involves Rapid Dynamin-Regulated Clathrin-Mediated Endocytosis, Followed by Slower Intracellular Trafficking. J. Virol. 2000, 74, 1919–1930. [Google Scholar] [CrossRef]
- Suikkanen, S.; Antila, M.; Jaatinen, A.; Vihinen-Ranta, M.; Vuento, M. Release of Canine Parvovirus from Endocytic Vesicles. Virology 2003, 316, 267–280. [Google Scholar] [CrossRef]
- Belmonte, S.A.; Mayorga, L.S.; Tomes, C.N. The Molecules of Sperm Exocytosis. In Advances in Anatomy Embryology and Cell Biology; Springer: Zurich, Switzerland, 2016; Volume 220, pp. 71–92. ISBN 9783319305677. [Google Scholar]
- Oresti, G.M.; Peñalva, D.A.; Luquez, J.M.; Antollini, S.S.; Aveldaño, M.I. Lipid Biochemical and Biophysical Changes in Rat Spermatozoa during Isolation and Functional Activation in Vitro1. Biol. Reprod. 2015, 93, 1–13. [Google Scholar] [CrossRef]
- Ahumada-Gutierrez, H.; Peñalva, D.A.; Enriz, R.D.; Antollini, S.S.; Cascales, J.J.L. Mechanical Properties of Bilayers Containing Sperm Sphingomyelins and Ceramides with Very Long-Chain Polyunsaturated Fatty Acids. Chem. Phys. Lipids 2019, 218, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G. Lipids, Curvature, and Nano-Medicine. Eur. J. Lipid Sci. Technol. 2011, 113, 1174–1187. [Google Scholar] [CrossRef]
- Fanani, M.L.; Nocelli, N.E.; Zulueta Díaz, Y. de las M. What Can We Learn about Amphiphile-Membrane Interaction from Model Lipid Membranes? Biochim. Biophys. Acta-Biomembr. 2022, 1864, 183781. [Google Scholar] [CrossRef] [PubMed]
- Lucio, M.; Lima, J.L.F.C.; Reis, S. Drug-Membrane Interactions: Significance for Medicinal Chemistry. Curr. Med. Chem. 2010, 17, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Miettinen, M.S.; Fricke, N.; Lipowsky, R.; Dimova, R. The Glycolipid GM1 Reshapes Asymmetric Biomembranes and Giant Vesicles by Curvature Generation. Proc. Natl. Acad. Sci. USA 2018, 115, 5756–5761. [Google Scholar] [CrossRef]
- Dymond, M.; Attard, G.; Postle, A.D. Testing the Hypothesis That Amphiphilic Antineoplastic Lipid Analogues Act through Reduction of Membrane Curvature Elastic Stress. J. R. Soc. Interface 2008, 5, 1371–1386. [Google Scholar] [CrossRef]
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; De Vries, P.J. Miltefosine: A Review of Its Pharmacology and Therapeutic Efficacy in the Treatment of Leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- Zulueta Díaz, Y.d.l.M.; Ambroggio, E.E.; Fanani, M.L. Miltefosine Inhibits the Membrane Remodeling Caused by Phospholipase Action by Changing Membrane Physical Properties. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183407. [Google Scholar] [CrossRef] [PubMed]
- Rakotomanga, M.; Blanc, S.; Gaudin, K.; Chaminade, P.; Loiseau, P.M. Miltefosine Affects Lipid Metabolism in Leishmania Donovani Promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1425–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Lipid Abbreviation and Name) * | Chemical Structure ** | Comments |
|---|---|---|
| CER; ceramide |  The structure corresponds to PCER (N-hexadecanoyl-D-erythro-sphingosine) | Product of SMase. |
| DAG, diacylglycerol |  The structure corresponds to DOG (1-2-dioleoyl-sn-glycerol) | Product of PLC. |
| FFA; free fatty acid |  The structure corresponds to arachidonic acid (AA) or 5,8,11,14-all-cis-Eicosatetraenoic acid | Product of PLA2. |
| LysoPC; lysophosphatidylcholine |  The structure corresponds to 2-oleoyl-sn-glycero-3-phosphocholine | Product of PLA2. |
| PA; phosphatidic acid |  The structure corresponds to DOPA (1,2-dioleoyl-sn-glycero-3-phosphate, sodium salt) | Product of Phospholipase D (PLD). |
| PC; phosphatidylcholine |  The structure corresponds to DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) | Substrate of Phospholipase A2 (PLA2) and Phospholipase C (PLC). |
| PE; phosphatidylethanolamine |  The structure corresponds to DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine) | |
| PIP2; phosphatidylinositol-4,5-bisphosphate |  The structure corresponds to 1,2-dioctanoyl-sn-glycero-3-phospho-(1′-myo-inositol-4′,5′-bisphosphate) (ammonium salt) | Substrate of PLC. |
| SM; sphingomyelin |  The structure corresponds to PSM (N-palmitoyl-D-erythro-sphingosylphosphorylcholine) | Substrate of Sphingomyelinase (SMase). |
| Sphingosine |  The structure corresponds to 2S, 3R(2S,3R,4E)-2-aminooctadec-4-ene-1,3-diol | |
| Sphingosine-1P |  The structure corresponds to D-erythro-sphingosine-1-phosphate | |
| V-CER; CER that contain N-linked very long long-chain polyunsaturated fatty acids (VLCPUFA). |  The structure corresponds to the CER containing an acyl chain C28:4. X = H or OH corresponding to n- or h- species, respectively. | Product of SMase |
| V-SM; SM that contains N-linked very long-chain polyunsaturated fatty acids (VLCPUFA) |  The structure corresponds to the SM containing an acyl chain C32:5. X = H or OH corresponding to n- or h- species, respectively. | Substrate of SMase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanani, M.L.; Ambroggio, E.E. Phospholipases and Membrane Curvature: What Is Happening at the Surface? Membranes 2023, 13, 190. https://doi.org/10.3390/membranes13020190
Fanani ML, Ambroggio EE. Phospholipases and Membrane Curvature: What Is Happening at the Surface? Membranes. 2023; 13(2):190. https://doi.org/10.3390/membranes13020190
Chicago/Turabian StyleFanani, María Laura, and Ernesto Esteban Ambroggio. 2023. "Phospholipases and Membrane Curvature: What Is Happening at the Surface?" Membranes 13, no. 2: 190. https://doi.org/10.3390/membranes13020190
APA StyleFanani, M. L., & Ambroggio, E. E. (2023). Phospholipases and Membrane Curvature: What Is Happening at the Surface? Membranes, 13(2), 190. https://doi.org/10.3390/membranes13020190