pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release?
Abstract
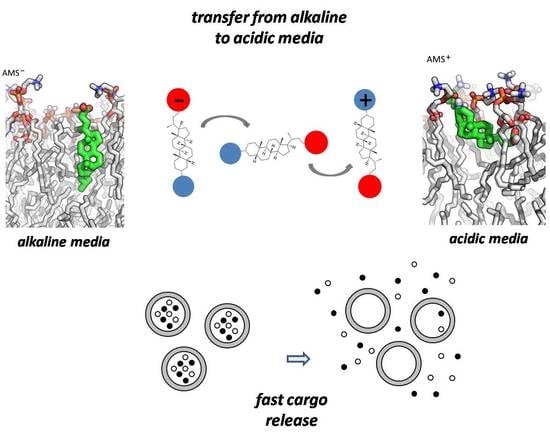
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Liposomes
2.3. Methods
2.4. Molecular Dynamic (MD) Simulations
2.5. ATR-FTIR Spectroscopy Experiments
3. Results and Discussion
3.1. POPC/AMS Liposomes
3.2. Release of the Cargo by POPC/AMS Liposomes under the Changes in the pH
3.3. Molecular Modeling of the Switch
3.4. ATR-FTIR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.M.; Whitehead, K.A. In pursuit of a moving target: Nanotherapeutics for the treatment of non-Hodgkin B-cell lymphoma. Expert Opin. Drug Deliv. 2014, 11, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Carvalho, M.A.; Castanheira, E.M.S. Functionalized Liposome and Albumin-Based Systems as Carriers for Poorly Water-Soluble Anticancer Drugs: An Updated Review. Biomedicines 2022, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Europ. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Pereira, F.H.; Batalhão, M.E.; Cárnio, E.C. Correlation between body temperature, blood pressure and plasmatic nitric oxide in septic patients. Rev. Lat. Am. Enfermagem 2014, 22, 123–128. [Google Scholar] [CrossRef]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 2002, 8, 1284–1291. [Google Scholar] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V.P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annual Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Karanth, H.; Murthy, R.S. pH-sensitive liposomes--principle and application in cancer therapy. J. Pharm. Pharmacol. 2007, 59, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.D.S.; Lopes, S.C.; Franco, M.S.; Oliveira, M.C. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef]
- Aryasomayajula, B.; Salzano, G.; Torchilin, V.P. Multifunctional Liposomes. Methods Mol. Biol. 2017, 1530, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gagne, L.; Chen, H.; Szoka, F.C. Novel ortho ester-based, pH-sensitive cationic lipid for gene delivery in vitro and in vivo. J. Liposome Res. 2014, 24, 90–98. [Google Scholar] [CrossRef]
- Viricel, W.; Mbarek, A.; Leblond, J. Switchable Lipids: Conformational Change for Fast pH-Triggered Cytoplasmic Delivery. Angew. Chem. Int. Ed. 2015, 54, 12743–12747. [Google Scholar] [CrossRef] [Green Version]
- Samoshina, N.M.; Liu, X.; Brazdova, B.; Franz, A.H.; Samoshin, V.V.; Guo, X. Fliposomes: pH-Sensitive Liposomes Containing a trans-2-morpholinocyclohexanol-Based Lipid That Performs a Conformational Flip and Triggers an Instant Cargo Release in Acidic Medium. Pharmaceutics 2011, 3, 379–405. [Google Scholar] [CrossRef] [Green Version]
- Yaroslavov, A.; Efimova, A.; Smirnova, N.; Erzunov, D.; Lukashev, N.; Grozdova, I.; Melik-Nubarov, N. A novel approach to a controlled opening of liposomes. Colloids Surf. B Biointerfaces 2020, 190, 110906. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.S.; Efimova, A.A.; Kazantsev, A.V.; Erzunov, D.A.; Lukashev, N.V.; Grozdova, I.D.; Melik-Nubarov, N.S.; Yaroslavov, A.A. pH-Sensitive liposomes with embedded ampholytic derivatives of cholan-24-oic acid. Mendeleev Commun. 2021, 31, 827–829. [Google Scholar] [CrossRef]
- Gurtovenko, A.A. Molecular-Level Insight into the Interactions of DNA/Polycation Complexes with Model Cell Membranes. J. Phys. Chem. B 2019, 123, 6505–6514. [Google Scholar] [CrossRef]
- Semenyuk, P.I.; Efimova, A.A.; Lentin, I.I.; Le-Deygen, I.M.; Izumrudov, V.A. Interaction of Ionenes with Lipid Membrane: Unusual Impact of Charge Density. Langmuir 2020, 36, 14717–14727. [Google Scholar] [CrossRef] [PubMed]
- Kostritskii, A.Y.; Kondinskaia, D.A.; Nesterenko, A.M.; Gurtovenko, A.A. Adsorption of Synthetic Cationic Polymers on Model Phospholipid Membranes: Insight from Atomic-Scale Molecular Dynamics Simulations. Langmuir 2016, 32, 10402–10414. [Google Scholar] [CrossRef]
- Trosheva, K.S.; Sorokina, S.A.; Efimova, A.A.; Semenyuk, P.I.; Berkovich, A.K.; Yaroslavov, A.A.; Shifrina, Z.B. Interaction of multicomponent anionic liposomes with cationic pyridylphenylene dendrimer: Does the complex behavior depend on the liposome composition? Biochim. Biophys. Acta Biomembr. 2021, 1863, 183761. [Google Scholar] [CrossRef]
- Wilkosz, N.; Jamróz, D.; Kopeć, W.; Nakai, K.; Yusa, S.I.; Wytrwal-Sarna, M.; Bednar, J.; Nowakowska, M.; Kepczynski, M. Effect of Polycation Structure on Interaction with Lipid Membranes. J. Phys. Chem. B 2017, 121, 7318–7326. [Google Scholar] [CrossRef]
- Awasthi, N.; Kopec, W.; Wilkosz, N.; Jamróz, D.; Hub, J.S.; Zatorska, M.; Petka, R.; Nowakowska, M.; Kepczynski, M. Molecular Mechanism of Polycation-Induced Pore Formation in Biomembranes. ACS Biomater. Sci. Eng. 2019, 5, 780–794. [Google Scholar] [CrossRef] [Green Version]
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.-M. Attenuated Total Reflection Infrared Spectroscopy of Proteins and Lipids in Biological Membranes. Biochim. Biophys. Acta Rev. Biomembr. 1999, 1422, 105–185. [Google Scholar] [CrossRef]
- Biruss, B.; Dietl, R.; Valenta, C. The Influence of Selected Steroid Hormones on the Physicochemical Behaviour of DPPC Liposomes. Chem. Phys. Lipids. 2007, 148, 84–90. [Google Scholar] [CrossRef]
- Deygen, I.M.; Seidl, C.; Kölmel, D.K.; Bednarek, C.; Heissler, S.; Kudryashova, E.V.; Bräse, S.; Schepers, U. Novel Prodrug of Doxorubicin Modified by Stearoylspermine Encapsulated into PEG-Chitosan-Stabilized Liposomes. Langmuir 2016, 32, 10861–10869. [Google Scholar] [CrossRef] [PubMed]
- Le-Deygen, I.M.; Vlasova, K.Y.; Kutsenok, E.O.; Usvaliev, A.D.; Efremova, M.V.; Zhigachev, A.O.; Rudakovskaya, P.G.; Golovin, D.Y.; Gribanovsky, S.L.; Kudryashova, E.V.; et al. Magnetic nanorods for remote disruption of lipid membranes by non-heating low frequency magnetic field. Nanomedicine 2019, 21, 102065. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D., Jr. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, R.W.; Mackerell, A.D. Development of the CHARMM Force Field for Lipids. J. Phys. Chem. Lett. 2011, 2, 1526–1532. [Google Scholar] [CrossRef] [Green Version]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Garidel, P.; Suwalsky, M.; Howe, J.; Brandenburg, K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: A physico-chemical study with lecithin phospholipids. Biochim. Biophys. Acta 2009, 1788, 1296–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disalvo, E.A.; Frias, M.A. Water state and carbonyl distribution populations in confined regions of lipid bilayers observed by FTIR spectroscopy. Langmuir 2013, 29, 6969–6974. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N.; Pohle, W.; Mantsch, H.H. Components of the carbonyl stretching band in the infrared spectra of hydrated 1,2-diacylglycerolipid bilayers: A reevaluation. Biophys. J. 1994, 67, 2367–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobroff, V.; Rubio, C.; Vigier, V.; Petibois, C. FTIR spectroscopy characterization of fatty-acyl-chain conjugates. Anal. Bioanal. Chem. 2016, 408, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef] [Green Version]







| Amino Group | Carboxylic Group | Counterions | |
|---|---|---|---|
| AMS+_NH2top | >NH2+ | –COOH | 4x Na+, 20x Cl– |
| AMS+_COOtop | >NH2+ | –COOH | 4x Na+, 20x Cl– |
| AMS0_NH2top | >NH2+ | –COO– | 20x Na+, 20x Cl– |
| AMS0_COOtop | >NH2+ | –COO– | 20x Na+, 20x Cl– |
| AMS−_NH2top | >NH | –COO– | 20x Na+, 4x Cl– |
| AMS−_COOtop | >NH | –COO– | 20x Na+, 4x Cl– |
| Total Lipid Concentration, mg/mL | ||||||
|---|---|---|---|---|---|---|
| 2 | 1 | 0.8 | 0.6 | 0.4 | 0.2 | |
| EPM, (μm/s)/(V/cm) | −1.61 ± 0.11 | −1.61 ± 0.11 | −1.58 ± 0.08 | −1.59 ± 0.07 | −1.62 ± 0.08 | −1.63 ± 0.06 |
| Hydrodynamic diameter, nm | 45 ± 5 | 42 ± 4 | 39 ± 2 | 44 ± 2 | 39 ± 4 | 43 ± 5 |
| Total Lipid Concentration, mg/mL | ||||||
|---|---|---|---|---|---|---|
| 2 | 1 | 0.8 | 0.6 | 0.4 | 0.2 | |
| EPM, (μm/s)/(V/cm) | 2.97 ± 0.07 | 2.98 ± 0.05 | 2.98 ± 0.06 | 2.98 ± 0.07 | 2.99 ± 0.08 | 3.00 ± 0.07 |
| Hydrodynamic diameter, nm | 36 ± 6 | 39 ± 5 | 40 ± 3 | 35 ± 3 | 47 ± 3 | 36 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, A.A.; Popov, A.S.; Kazantsev, A.V.; Semenyuk, P.I.; Le-Deygen, I.M.; Lukashev, N.V.; Yaroslavov, A.A. pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release? Membranes 2023, 13, 407. https://doi.org/10.3390/membranes13040407
Efimova AA, Popov AS, Kazantsev AV, Semenyuk PI, Le-Deygen IM, Lukashev NV, Yaroslavov AA. pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release? Membranes. 2023; 13(4):407. https://doi.org/10.3390/membranes13040407
Chicago/Turabian StyleEfimova, Anna A., Anton S. Popov, Alexey V. Kazantsev, Pavel I. Semenyuk, Irina M. Le-Deygen, Nikolay V. Lukashev, and Alexander A. Yaroslavov. 2023. "pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release?" Membranes 13, no. 4: 407. https://doi.org/10.3390/membranes13040407
APA StyleEfimova, A. A., Popov, A. S., Kazantsev, A. V., Semenyuk, P. I., Le-Deygen, I. M., Lukashev, N. V., & Yaroslavov, A. A. (2023). pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release? Membranes, 13(4), 407. https://doi.org/10.3390/membranes13040407