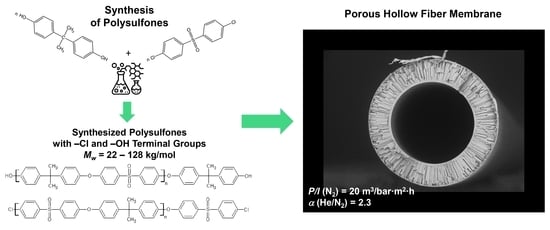
Effect of Molecular Weight and Chemical Structure of Terminal Groups on the Properties of Porous Hollow Fiber Polysulfone Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PSF with Different Terminal Groups
2.3. Study of Synthesized PSFs
2.3.1. Nuclear Magnetic Resonance (NMR) Method
2.3.2. Differential Scanning Calorimetry Method
2.3.3. Gel Permeation Chromatography Method
2.3.4. Determination of Coagulation Values
2.4. Dope Solution Preparation
2.5. Polymer Solution Viscosity Measurement
2.6. Study of the Phase Inversion Kinetics
2.7. Hollow Fiber Membranes Preparation
2.8. Study of PSF Hollow Fiber Membranes
2.8.1. Gas Transport Properties
2.8.2. Porosimetry
2.8.3. Scanning Electron Microscopy
3. Results and Discussion
3.1. Properties of Synthesized PSFs
3.2. Properties of Dope Solutions
3.3. Study of Porous Hollow Fiber Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, C.; Khulbe, K.; Matsuura, T.; Ismail, A. Recent progresses in polymeric hollow fiber membrane preparation, characterization and applications. Sep. Purif. Technol. 2013, 111, 43–71. [Google Scholar] [CrossRef]
- Kheirieh, S.; Asghari, M.; Afsari, M. Application and modification of polysulfone membranes. Rev. Chem. Eng. 2018, 34, 657–693. [Google Scholar] [CrossRef]
- Serbanescu, O.; Voicu, S.; Thakur, V. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Ham, M.H.; Kim, I.S. Novel sulfonated graphene oxide incorporated polysulfone nanocomposite membranes for enhanced-performance in ultrafiltration process. Chemosphere 2018, 207, 581–589. [Google Scholar] [CrossRef]
- Khulbe, K.; Matsuura, T. Thin Film Composite and/or Thin Film Nanocomposite Hollow Fiber Membrane for Water Treatment, Pervaporation, and Gas/Vapor Separation. Polymers 2018, 10, 1051. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Hou, Z.; Liu, Z.; Bian, X.; Shi, L.; Li, L. Effect of polyethersulfone molecular weight on structure and performance of ultrafiltration membranes. Ind. Eng. Chem. Res. 2010, 49, 9988–9997. [Google Scholar] [CrossRef]
- Hołda, A.; Roeck, M.; Katrien, H.; Vankelecom, I. The influence of polymer purity and molecular weight on the synthesis of integrally skinned polysulfone membranes. J. Membr. Sci. 2013, 446, 113–120. [Google Scholar] [CrossRef]
- Tian, X.; Qiu, Y. 2-methoxyethylacrylate modified polysulfone membrane and its blood compatibility. Arch. Biochem. Biophys. 2017, 631, 49–57. [Google Scholar] [CrossRef]
- Lin, X.; Kim, S.; Shamsaei, E.; Xu, T.; Fang, X.; Wang, H. Preparation of porous diffusion dialysis membranes by functionalization of polysulfone for acid recovery. J. Membr. Sci. 2017, 524, 557–564. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Qiu, Y. A novel kind of polysulfone material with excellent biocompatibility modified by the sulfonated hydroxypropyl chitosan. Mater. Sci. Eng. C 2017, 79, 570–580. [Google Scholar] [CrossRef]
- Hu, B.; Miao, L.; Zhao, Y.; Lü, C. Azide-assisted crosslinked quaternized polysulfone with reduced graphene oxide for highly stable anion exchange membranes. J. Membr. Sci. 2017, 530, 84–94. [Google Scholar] [CrossRef]
- Venugopal, K.; Dharmalingam, S. Desalination efficiency of a novel bipolar membrane based on functionalized polysulfone. Desalination 2012, 296, 37–45. [Google Scholar] [CrossRef]
- Camacho-Zuñiga, C.; Ruiz-Treviño, F.; Hernández-López, S.; Zolotukhin, M.; Maurer, F.; González-Montiel, A. Aromatic polysulfone copolymers for gas separation membrane applications. J. Membr. Sci. 2009, 340, 221–226. [Google Scholar] [CrossRef]
- Martínez-Morlanes, M.; Martos, A.; Varez, A.; Levenfeld, B. Synthesis and characterization of novel hybrid polysulfone/silica membranes doped with phosphomolybdic acid for fuel cell applications. J. Membr. Sci. 2015, 492, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Gumí, T.; Minguillón, C.; Palet, C. Separation of propranolol enantiomers through membranes based on chiral derivatized polysulfone. Polymer 2005, 46, 12306–12312. [Google Scholar] [CrossRef]
- Abu-Thabit, N.; Ali, S.; Zaidi, S. New highly phosphonated polysulfone membranes for PEM fuel cells. J. Membr. Sci. 2010, 360, 26–33. [Google Scholar] [CrossRef]
- Celebi, O.; Lee, C.; Lin, Y.; McGrath, J.; Riffle, J. Synthesis and characterization of polyoxazoline–polysulfone triblock copolymers. Polymer 2011, 52, 4718–4726. [Google Scholar] [CrossRef]
- Du, X.; Meng, J.; Xu, R.; Shi, Q.; Zhang, Y. Polyol-grafted polysulfone membranes for boron removal: Effects of the ligand structure. J. Membr. Sci. 2015, 476, 205–215. [Google Scholar] [CrossRef]
- Jujie, L.; He, X.; Si, Z. Polysulfone membranes containing ethylene glycol monomers: Synthesis, characterization, and CO 2/CH 4 separation. J. Polym. Res. 2017, 24, 1–14. [Google Scholar] [CrossRef]
- Xie, Y.; Moreno, N.; Calo, V.; Cheng, H.; Hong, P.; Sougrat, R.; Behzad, A.; Tayouo, R.; Nunes, S. Synthesis of highly porous poly (tert-butyl acrylate)-b-polysulfone-b-poly (tert-butyl acrylate) asymmetric membranes. Polym. Chem. 2016, 7, 3076–3089. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, L.; Yi, Z.; Zhu, B.; Xu, Y. Improving the hydrophilicity and fouling-resistance of polysulfone ultrafiltration membranes via surface zwitterionicalization mediated by polysulfone-based triblock copolymer additive. J. Membr. Sci. 2013, 440, 40–47. [Google Scholar] [CrossRef]
- Dolatkhah, F.; Mohammadi, T.; Tofighy, M. Polysulfone hollow fiber membrane containing charcoal-carbon nanomaterial for wastewater treatment in membrane bioreactor. J. Water Process. Eng. 2022, 50, 103222. [Google Scholar] [CrossRef]
- Sasikumar, B.; Bisht, S.; Arthanareeswaran, G.; Ismail, A.; Othman, M. Performance of polysulfone hollow fiber membranes encompassing ZIF-8, SiO2/ZIF-8, and amine-modified SiO2/ZIF-8 nanofillers for CO2/CH4 and CO2/N2 gas separation. Sep. Purif. Technol. 2021, 264, 118471. [Google Scholar] [CrossRef]
- Khan, I.; Othman, M.; Jilani, A.; Ismail, A.; Hashim, H.; Jaafar, J.; Zulhairun, A.; Rahman, M.; Rehman, G. ZIF-8 based polysulfone hollow fiber membranes for natural gas purification. Polym. Test 2020, 84, 106415. [Google Scholar] [CrossRef]
- Said, N.; Hasbullah, H.; Abidin, M.; Ismail, A.; Goh, P.; Othman, M.; Kadir, S.; Kamal, F.; Abdullah, M.; Ng, B. Facile modification of polysulfone hollow-fiber membranes via the incorporation of well-dispersed iron oxide nanoparticles for protein purification. J. Appl. Polym. Sci. 2019, 136, 47502. [Google Scholar] [CrossRef]
- Wijiyanti, R.; Ubaidillah, A.; Gunawan, T.; Karim, Z.; Ismail, A.; Smart, S.; Lin, R.; Widiastuti, N. Polysulfone mixed matrix hollow fiber membranes using zeolite templated carbon as a performance enhancement filler for gas separation. Chem. Eng. Res. Des. 2019, 150, 274–288. [Google Scholar] [CrossRef]
- Said, N.; Mansur, S.; Abidin, M.N.Z.; Ismail, A.F. Fabrication and Characterization of Polysulfone/Iron Oxide Nanoparticle Mixed Matrix Hollow Fiber Membranes for Hemodialysis: Effect of Dope Extrusion Rate and Air Gap. J. Membr. Sci. Res. 2023, 9, 1–7. [Google Scholar] [CrossRef]
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; Keong, L.K. Issues and Current Trends of Hollow-Fiber Mixed-Matrix Membranes for CO2 Separation from N2 and CH4. Chem. Eng. Technol. 2018, 41, 235–252. [Google Scholar] [CrossRef]
- Siddique, T.; Gangadoo, S.; Quang Pham, D.; Dutta, N.K.; Choudhury, N.R. Antifouling and Antimicrobial Study of Nanostructured Mixed-Matrix Membranes for Arsenic Filtration. Nanomaterials 2023, 13, 738. [Google Scholar] [CrossRef]
- Plisko, T.; Bildyukevich, A.; Zhao, L.; Huang, W.; Volkov, V.; Huang, Z. Formation of Polysulfone Hollow Fiber Membranes Using the Systems with Lower Critical Solution Temperature. Fibers 2021, 9, 28. [Google Scholar] [CrossRef]
- Sengur-Tasdemir, R.; Urper-Bayram, G.; Turken, T.; Ates-Genceli, E.; Tarabara, V.; Koyuncu, I. Hollow fiber nanofiltration membranes for surface water treatment: Performance evaluation at the pilot scale. J. Water Process. Eng. 2021, 42, 102100. [Google Scholar] [CrossRef]
- Lim, Y.; Bak, C.; Kim, Y. Comprehensive experimental and theoretical insights into the performance of polysulfone hollow-fiber membrane modules in biogas purification process. Chem. Eng. J. 2022, 433, 134616. [Google Scholar] [CrossRef]
- Mansur, S.; Othman, M.; Abidin, M.; Ismail, A.; Kadir, S.A.; Goh, P.; Hasbullah, H.; Ng, B.; Abdullah, M.; Mustafar, R. Enhanced adsorption and biocompatibility of polysulfone hollow fibre membrane via the addition of silica/alpha-mangostin hybrid nanoparticle for uremic toxins removal. J. Environ. Chem. Eng. 2021, 9, 106141. [Google Scholar] [CrossRef]
- Peechmani, P.; Othman, M.; Kamaludin, R.; Puteh, M.; Jaafar, J.; Rahman, M.; Ismail, A.; Kadir, S.A.; Illias, R.; Gallagher, J.; et al. High flux polysulfone braided hollow fiber membrane for wastewater treatment role of zinc oxide as hydrophilic enhancer. J. Environ. Chem. Eng. 2021, 9, 105873. [Google Scholar] [CrossRef]
- Kurdanova, Z. Synthesis and Properties of Polyphenylene Sulfone and Its Copolymers for Use in Additive Technologies. Ph.D. Thesis, Kabardino-Balkarian State University, Nalchik, Russia, 2017. [Google Scholar]
- Grushevenko, E.A.; Borisov, I.L.; Knyazeva, A.A.; Volkov, V.V.; Volkov, A.V. Polyalkylmethylsiloxanes composite mem-branes for hydrocarbon/methane separation: Eight component mixed-gas permeation properties. Sep. Purif. Technol. 2020, 241, 116696. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Bakhtin, D.S.; Bondarenko, G.N.; Levin, I.S.; Volkov, A.V. Silicone rubbers with alkyl side groups for C3+ hydrocarbon separation. React. Funct. Polym. 2019, 134, 156–165. [Google Scholar] [CrossRef]
- Plisko, T.; Bildyukevich, A.; Karslyan, Y.; Ovcharova, A.; Volkov, V. Development of high flux ultrafiltration polyphenylsulfone membranes applying the systems with upper and lower critical solution temperatures: Effect of polyethylene glycol molecular weight and coagulation bath temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Ovcharova, A.; Vasilevsky, V.; Borisov, I.; Bazhenov, S.; Volkov, A.; Bildyukevich, A.; Volkov, V. Polysulfone porous hollow fiber membranes for ethylene-ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2017, 183, 162–172. [Google Scholar] [CrossRef]
- Malakhov, A.; Bazhenov, S.; Vasilevsky, V.; Borisov, I.; Ovcharova, A.; Bildyukevich, A.; Volkov, V.; Giorno, L.; Volkov, A. Thin-film composite hollow fiber membranes for ethylene/ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2019, 219, 64–73. [Google Scholar] [CrossRef]
- Borisov, I.; Ovcharova, A.; Bakhtin, D.; Bazhenov, S.; Volkov, A.; Ibragimov, R.; Gallyamov, R.; Bondarenko, G.; Mozhchil, R.; Bildyukevich, A.; et al. Development of Polysulfone Hollow Fiber Porous Supports for High Flux Composite Membranes: Air Plasma and Piranha Etching. Fibers 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Borisov, I.; Vasilevsky, V.; Matveev, D.; Ovcharova, A.; Volkov, A.; Volkov, V. Effect of Temperature Exposition of Casting Solution on Properties of Polysulfone Hollow Fiber Membranes. Fibers 2019, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Anokhina, T.; Borisov, I.; Yushkin, A.; Vaganov, G.; Didenko, A.; Volkov, A. Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties. Polymers 2020, 12, 2785. [Google Scholar] [CrossRef] [PubMed]
- Matveev, D.; Kutuzov, K.; Vasilevsky, V. Effect of Draw Ratio on the Morphology of Polysulfone Hollow Fiber Membranes. Membr. Technol. 2020, 2, 351–356. [Google Scholar] [CrossRef]
- Korshak, V.; Vinogradova, S. Nonequilibrium polycondensation. Polym. Sci. USSR 1972, 13, 415–434. [Google Scholar] [CrossRef]
- Kalugina, E.V. Thermal Transformations and Stabilization of Some Heat-Resistant Heterochain Polymers. Ph.D. Thesis, Semenov Institute of Chemical Physics, Moscow, Russia, 2003. [Google Scholar]
- Zhansitov, A.; Khashirova, S.; Slonov, A.; Kurdanova, Z.; Shabaev, A.; Khashirov, A.; Mikitaev, A. Development of technology of polysulfone production for 3D printing. High Perform. Polym. 2017, 29, 724–729. [Google Scholar] [CrossRef]
- Shabaev, A.; Zhansitov, A.; Kurdanova, Z.; Khashirova, S.; Mikitaev, A. New method of investigation of polysulfone thermal destruction. Polym. Sci. Ser. B+ 2017, 59, 216–224. [Google Scholar] [CrossRef]
- Hoffmann, T.; Pospiech, D.; Kretzschmar, B.; Reuter, U.; Häußler, L.; Eckert, F.; Perez-Graterol, R.; Sandler, J.K.; Altstädt, V. Modification of Polysulfones by Carboxylic Acids. High Perform. Polym. 2007, 19, 48–61. [Google Scholar] [CrossRef]
- Matveev, D.; Borisov, I.; Vasilevsky, V.; Karpacheva, G.; Volkov, V. Spinning of Polysulfone Hollow Fiber Membranes Using Constant Dope Solution Composition: Viscosity Control via Temperature. Membranes 2022, 12, 1257. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, S.; Hong, Z.; Ma, H.; Liu, C.; Shao, W. Effects of Casting Solvents on the Morphologies, Properties, and Performance of Polysulfone Supports and the Resultant Graphene Oxide-Embedded Thin-Film Nanocomposite Nanofiltration Membranes. Ind. Eng. Chem. Res. 2018, 57, 16464–16475. [Google Scholar] [CrossRef]
- Thuyavan, Y.; Anantharaman, N.; Arthanareeswaran, G.; Ismail, A. Impact of solvents and process conditions on the formation of polyethersulfone membranes and its fouling behavior in lake water filtration. J. Chem. Technol. Biotechnol. 2016, 91, 2568–2581. [Google Scholar] [CrossRef]
- Liu, Y.; Koops, G.; Strathmann, H. Characterization of morphology controlled polyethersulfone hollow fiber membranes by the addition of polyethylene glycol to the dope and bore liquid solution. J. Membr. Sci. 2003, 223, 187–199. [Google Scholar] [CrossRef]







| Functional Group | PSF Supplier | PSF MM, kg/mol | Membrane Configuration | Application | Ref. |
|---|---|---|---|---|---|
| 2-methoxyethyl acrylate | Sigma-Aldrich, China | Mn ~22 | Flat-sheet | Hemodialysis | [8] |
| quaternary ammonium groups N(CH3)3+-CH2– | Sigma-Aldrich, Australia | Mw ~35 | Flat-sheet | Dialysis | [9] |
| chitosan | Sigma-Aldrich, St. Louis, MO, USA | Mn ~22 | Flat-sheet | Hemodialysis | [10] |
| azide groups N3-(CH2)3-N(CH3)2+-CH2– | Sigma-Aldrich, China | Mw ~58 | Flat-sheet | Anion exchange membranes | [11] |
| –SO3H groups | Sigma-Aldrich, St. Louis, MO, USA | Mw ~35 | Flat-sheet | Seawater desalination | [12] |
| naphthalene moieties, –CF3 and –CH3 groups | synthesis within the work | - | Flat-sheet | Gas separation | [13] |
| –SO3H groups | Sigma-Aldrich, St. Louis, MO, USA | Mn ~22 | Flat-sheet | Polymer fuel cells | [14] |
| N-dodecyl-4(R)-hydroxy-l-proline | BASF, Ludwigshafen, Germany | - | Flat-sheet | Dialysis | [15] |
| phosphonic acid (HO)2(O)PH2C– | Sigma-Aldrich, St. Louis, MO, USA | Mw ~35 | Flat-sheet | Ion exchange membrane | [16] |
| 2-ethyl-2-oxazoline | synthesis within the work | Mn ~15.2 | Flat-sheet | Water purification | [17] |
| polymethacrylates | Solvay Advanced Polymers, Düsseldorf, Germany | Mn ~29 | Flat-sheet | Boron removal | [18] |
| CF3-groups, poly(ethylene glycol) | synthesis within the work | Mn ~5.2 Mw ~28.6 | Flat-sheet | CO2/CH4 separation | [19] |
| poly(tertbutylacrylate) | Sigma-Aldrich, St. Louis, MO, USA | Mn ~22 | Flat-sheet | UF | [20] |
| poly(N,N-dimethylamino-2-ethylmethacrylate) | Solvay Advanced Polymers | Mw ~17.6 | Flat-sheet | Hemodialysis | [21] |
| PSF | Solvent | Molar Ratio DCDPS: Bisphenol A |
|---|---|---|
| 1 | DMAc | 1:1.01 |
| 2 | 1:1.015 | |
| 3 | 1:1.025 | |
| 4 | 1.01:1 | |
| 5 | 1.018:1 | |
| 6 | 1.03:1 | |
| 7 | 1:1 | |
| 8 | NMP | 1:1 |
| 9 | DMSO | 1:1 |
| PSF | (-OH):(-Cl) | Mw, kg/mol | Mn, kg/mol | Mw/Mn | Estimated Number of Links | MNMR, kg/mol |
|---|---|---|---|---|---|---|
| 1 | 2.6:1 | 65 | 27 | 2.4 | 56 | 28 |
| 2 | 2.7:1 | 58 | 20 | 2.9 | 50 | 25 |
| 3 | 6.9:1 | 57 | 12 | 4.8 | 41 | 20 |
| 4 | 1:1.6 | 128 | 34 | 3.8 | 60 | 30 |
| 5 | 1:2.7 | 43 | 18 | 2.4 | 49 | 24 |
| 6 | 1:3.4 | 37 | 12 | 3.1 | 39 | 20 |
| 7 | 1.2:1 | 55 | 28 | 2.0 | 79 | 39 |
| 8 | 1.3:1 | 79 | 33 | 2.4 | 92 | 45 |
| 9 | 1.2:1 | 22 | 4 | 5.8 | 10 | 5 |
| Sample | η, Pa·s | υ, µm/s |
|---|---|---|
| PSF-1 | 32 | 4.9 |
| PSF-7 | 15 | 6.0 |
| PSF-8 | 56 | 3.9 |
| Sample | Dout, mm | δ, µm | P/l (N2), (m3/bar·m2·h) | P/l (He), (m3/bar·m2·h) | α (He/N2) | dmax, nm | dMFP, nm |
|---|---|---|---|---|---|---|---|
| PSF-1 | 0.8 | 150 | 20 | 45 | 2.3 | 28 | 23 |
| PSF-7 | 0.9 | 200 | 45 | 73 | 1.6 | 81 | 27 |
| PSF-8 | 0.8 | 100 | 1.2 | 2.3 | 1.9 | 35 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveev, D.; Raeva, A.; Borisov, I.; Vasilevsky, V.; Matveeva, Y.; Zhansitov, A.; Khashirova, S.; Volkov, V. Effect of Molecular Weight and Chemical Structure of Terminal Groups on the Properties of Porous Hollow Fiber Polysulfone Membranes. Membranes 2023, 13, 412. https://doi.org/10.3390/membranes13040412
Matveev D, Raeva A, Borisov I, Vasilevsky V, Matveeva Y, Zhansitov A, Khashirova S, Volkov V. Effect of Molecular Weight and Chemical Structure of Terminal Groups on the Properties of Porous Hollow Fiber Polysulfone Membranes. Membranes. 2023; 13(4):412. https://doi.org/10.3390/membranes13040412
Chicago/Turabian StyleMatveev, Dmitry, Alisa Raeva, Ilya Borisov, Vladimir Vasilevsky, Yulia Matveeva, Azamat Zhansitov, Svetlana Khashirova, and Vladimir Volkov. 2023. "Effect of Molecular Weight and Chemical Structure of Terminal Groups on the Properties of Porous Hollow Fiber Polysulfone Membranes" Membranes 13, no. 4: 412. https://doi.org/10.3390/membranes13040412
APA StyleMatveev, D., Raeva, A., Borisov, I., Vasilevsky, V., Matveeva, Y., Zhansitov, A., Khashirova, S., & Volkov, V. (2023). Effect of Molecular Weight and Chemical Structure of Terminal Groups on the Properties of Porous Hollow Fiber Polysulfone Membranes. Membranes, 13(4), 412. https://doi.org/10.3390/membranes13040412