Structural Impact of Selected Retinoids on Model Photoreceptor Membranes
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Lipids
2.2. All-Trans Retinal Dimer Synthesis
2.3. Preparation of Liposomes
2.4. EPR Measurements
2.5. Statistical Analysis
2.6. Molecular Dynamics Simulation
3. Results and Discuss
3.1. Plasma Membrane
3.2. Young Disc Membranes
3.3. Old Disc Membranes
3.4. Correlation Times
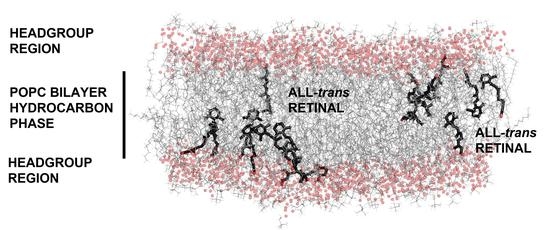
3.5. Molecular Dynamic Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, R.E.; Maude, M.B. Phospholipids of bovine outer segments. Biochemistry 1970, 9, 3624–3628. [Google Scholar] [CrossRef] [PubMed]
- Borggreven, J.M.P.M.; Daemen, F.J.M.; Bonting, S.L. Biochemical aspects of the visual process: VI. The lipid composition of native and hexane-extracted cattle rod outer segments. Biochim. Et Biophys. Acta (BBA) Lipids Lipid Metab. 1970, 202, 374–381. [Google Scholar] [CrossRef]
- Terrasa, A.M.; Guajardo, M.H.; Marra, C.A.; Zapata, G. α-Tocopherol protects against oxidative damage to lipids of the rod outer segments of the equine retina. Vet. J. 2009, 182, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.E.; Bazan, N.G.; Feeney-Burns, L.; Berman, E.R. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1433–1443. [Google Scholar]
- Albert, A.D.; Young, J.E.; Paw, Z. Phospholipid fatty acyl spatial distribution in bovine rod outer segment disk membranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 1998, 1368, 52–60. [Google Scholar] [CrossRef]
- Boesze-Battaglia, K.; Albert, A.D. Phospholipid distribution among bovine rod outer segment plasma membrane and disk membranes. Exp. Eye Res. 1992, 54, 821–823. [Google Scholar] [CrossRef]
- Boesze-Battaglia, K.J.; Fliesler, S.J.; Albert, A.D. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J. Biol. Chem. 1990, 265, 18867–18870. [Google Scholar] [CrossRef]
- Seregard, J.S.; Algvere, P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar]
- Rozanowska, M.; Sarna, T. Light-induced damage to the retina: Role of rhodopsin chromophore revisited. Photochem. Photobiol 2005, 81, 1305–1330. [Google Scholar] [CrossRef]
- Boulton, M.; Rozanowska, M.; Rozanowski, B. Retinal photodamage. J. Photochem. Photobiol. B 2001, 64, 144–161. [Google Scholar] [CrossRef]
- Damsgaard, C.; Country, M.W. The Opto-Respiratory Compromise: Balancing Oxygen Supply and Light Transmittance in the Retina. Physiology 2022, 37, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Imamoto, Y.; Shichida, Y. Cone visual pigments. Biochim. Biophys. Acta 2014, 1837, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Papermaster, D.S.; Dreyer, W.J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry 1974, 11, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.D.; Pugh, E.N. Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 2004, 23, 307–380. [Google Scholar] [CrossRef]
- Fukagawa, T.; Takafuji, K.; Tachibanaki, S.; Kawamura, S. Purification of cone outer segment for proteomic analysis on its membrane proteins in carp retina. PLoS ONE 2017, 12, e01739082017. [Google Scholar] [CrossRef]
- Gunkel, M.; Schöneberg, J.; Alkhaldi, W.; Irsen, S.H.; Noé, F.; Kaupp, U.B.; Al-Amoudi, A. Higher-order architecture of rhodopsin in intact photoreceptors and its implication for phototransduction kinetics. Structure 2015, 23, 628–638. [Google Scholar] [CrossRef]
- Pajares, M.A.; Rando, R.R. The active-site environment of rhodopsin. J. Biol. Chem. 1989, 264, 6804–6809. [Google Scholar] [CrossRef]
- Srinivasan, S.; Guixà-González, R.; Cordomí, A.; Garriga, P. Ligand Binding Mechanisms in Human Cone Visual Pigments. Trends Biochem. Sci. 2019, 44, 629–639. [Google Scholar] [CrossRef]
- Hayashi, S.; Tajkhorshid, E.; Schulten, K. Photochemical reaction dynamics of the primary event of vision studied by means of a hybrid molecular simulation. Biophys. J. 2009, 96, 403–416. [Google Scholar] [CrossRef]
- McBee, J.K.; Palczewski, K.; Baehr, W.; Pepperberg, D.R. Confronting Complexity: The Interlink of Phototransduction and Retinoid Metabolism in the Vertebrate Retina. Prog. Retin. Eye Res. 2001, 20, 469–529. [Google Scholar]
- Parker, R.O.; Crouch, R.K. Retinol dehydrogenases (RDHs) in the visual cycle. Exp. Eye Res. 2010, 91, 788–792. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, T.; Madigan, M.C.; Fernando, N.; Aggio-Bruce, R.; Zhou, F.; Pierce, M.; Chen, Y.; Huang, L.; Natoli, R.; et al. Interphotoreceptor Retinoid-Binding Protein (IRBP) in Retinal Health and Disease. Front. Cell. Neurosci. 2020, 14, 577935. [Google Scholar] [PubMed]
- Saari, J.C.; Garwin, G.G.; Van Hooser, J.P.; Palczewski, K. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vis. Res. 1998, 38, 1325–1333. [Google Scholar] [CrossRef]
- Maeda, T.; Golczak, M.; Maeda, A. Retinal photodamage mediated by all-trans-retinal. Photochem. Photobiol. 2012, 88, 1309–1319. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Handzel, K.; Boulton, M.E.; Rozanowski, B. Cytotoxicity of all-trans-retinal increases upon photodegradation. Photochem. Photobiol. 2012, 88, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Fishkin, N.E.; Sparrow, J.R.; Allikmets, R.; Nakanishi, K. Isolation and characterization of a retinal pigment epithelial cell fluorophore: An all-trans-retinal dimer conjugate. Proc. Natl. Acad. Sci. USA 2005, 102, 7091–7096. [Google Scholar] [CrossRef]
- Adler, L.; Boyer, N.P.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Chapter-Thirty-One-The 11-cis Retinal Origins of Lipofuscin in the Retina. In Progress in Molecular Biology and Translational Science; Hejtmancik, J.F., Nickerson, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. e1–e12. [Google Scholar]
- Katz, M.L.; Redmond, T.M. Effect of Rpe65 Knockout on Accumulation of Lipofuscin Fluorophores in the Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3023–3030. [Google Scholar]
- Parish, C.A.; Hashimoto, M.; Nakanishi, K.; Dillon, J.; Sparrow, J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 1998, 95, 14609–14613. [Google Scholar] [CrossRef]
- Mata, N.L.; Weng, J.; Travis, G. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. USA 2000, 97, 7154–7159. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Wu, Y.; Kim, C.Y.; Zhou, J. Phospholipid meets all-trans-retinal: The making of RPE bisretinoids. J. Lipid Res. 2010, 51, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Quazi, F.; Lenevich, S.; Molday, R. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nat. Commun. 2012, 3, 925. [Google Scholar]
- Gao, Z.; Liao, Y.; Chen, C.; Liao, C.; He, D.; Chen, J.; Ma, J.; Liu, Z.; Wu, Y. Conversion of all-trans-retinal into all-trans-retinal dimer reflects an alternative metabolic/antidotal pathway of all-trans-retinal in the retina. J. Biol. Chem. 2018, 293, 14507–14519. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Cai, X.; Xia, Q.; Chen, J.; Liao, Y.; Liu, Z.; Wu, Y. All-trans-retinal dimer formation alleviates the cytotoxicity of all-trans-retinal in human retinal pigment epithelial cells. Toxicology 2016, 37, 41–48. [Google Scholar] [CrossRef]
- Yoon, K.D.; Yamamoto, K.; Zhou, J.; Sparrow, J.R. Photo-products of retinal pigment epithelial bisretinoids react with cellular thiols. Mol. Vis. 2011, 17, 1839–1849. [Google Scholar]
- Yoon, K.D.; Yamamoto, K.; Ueda, K.; Zhou, J.; Sparrow, J.R. A Novel Source of Methylglyoxal and Glyoxal in Retina: Implications for Age-Related Macular Degeneration. PLoS ONE 2012, 7, e413092012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liao, Y.; Chen, J.; Dong, X.; Gao, Z.; Zhang, H.; Wu, X.; Liu, Z.; Wu, Y. Aberrant Buildup of All-Trans-Retinal Dimer, a Nonpyridinium Bisretinoid Lipofuscin Fluorophore, Contributes to the Degeneration of the Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1063–1075. [Google Scholar] [CrossRef]
- Fishkin, N.; Pescitelli, G.; Sparrow, J.R.; Nakanishi, K.; Berova, N. Absolute configurational determination of an all-trans-retinal dimer isolated from photoreceptor outer segments. Chirality 2004, 16, 637–641. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, T.; Imanishi, Y.; Kuksa, V.; Alekseev, A.; Bronson, J.D.; Zhang, H.; Zhu, L.; Sun, W.; Saperstein, D.A.; et al. Role of Photoreceptor-specific Retinol Dehydrogenase in the Retinoid Cycle in vivo. J. Biol. Chem. 2005, 280, 18822–18832. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kono, M.; Koutalos, Y. Photooxidation mediated by 11-cis and all-trans retinal in single isolated mouse rod photoreceptors. Photochem. Photobiol. Sci. 2020, 19, 1300–1307. [Google Scholar] [CrossRef]
- Sharma, R.; Schwarz, C.; Hunter, J.J.; Palczewska, G.; Palczewski, K.; Williams, D.R. Formation and Clearance of All-Trans-Retinol in Rods Investigated in the Living Primate Eye With Two-Photon Ophthalmoscopy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 604–613. [Google Scholar] [CrossRef]
- Chen, C.; Tsina, E.; Cornwall, M.C.; Crouch, R.K.; Vijayaraghavan, S.; Koutalos, Y. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors. Biophys. J. 2005, 88, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Verdegem, P.J.E.; Monnee, M.C.F.; Mulder, P.P.J.; Lugtenburg, J. Condensation of all-E-retinal. Tetrahedron. Lett. 1997, 38, 5355–5358. [Google Scholar] [CrossRef]
- Kusumi, A.; Pasenkiewicz-Gierula, M. Rotational diffusion of a steroid molecule in phosphatidylcholine membranes: Effects of alkyl chain length, unsaturation, and cholesterol as studied by a spin-label method. Biochemistry 1988, 27, 4407–4415. [Google Scholar] [CrossRef]
- Marsh, D. Electron Spin Resonance: Spin Labels. In Membrane Spectroscopy; Grell, E., Ed.; Springer: Berlin/Heidelberg, Germnay, 1981; pp. 51–142. [Google Scholar]
- Berliner, L.J. Spin labeling in enzymology: Spin-labeled enzymes and proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; pp. 418–480. [Google Scholar]
- Subczynski, W.K.; Wisniewska, A.; Yin, J.J.; Hyde, J.S.; Kusumi, A. Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry 1994, 33, 7670–7681. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim. Et Biophys. Acta (BBA) Biomembr. 1998, 1368, 235–246. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol: An open source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. Charmm-Gui: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Seelig, J.; Waespe-Sarcevic, N. Molecular order in cis and trans unsaturated phospholipid bilayers. Biochemistry 1978, 17, 3310–3315. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A Gen. Phys. 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Markiewicz, M.; Pasenkiewicz-Gierula, M. Comparative Model Studies of Gastric Toxicity of Nonsteroidal Anti-Inflammatory Drugs. Langmuir 2011, 27, 6950–6961. [Google Scholar] [CrossRef]
- Pasenkiewicz-Gierula, M.; Róg, T.; Grochowski, J.; Serda, P.; Czarnecki, R.; Librowski, T.; Lochyński, S. Effects of a Carane Derivative Local Anesthetic on a Phospholipid Bilayer Studied by Molecular Dynamics Simulation. Biophys. J. 2003, 85, 1248–1258. [Google Scholar] [CrossRef]
- Albert, A.; Alexander, D.; Boesze-Battaglia, K. Cholesterol in the rod outer segment: A complex role in a “simple” system. Chem. Phys. Lipids 2016, 199, 94–105. [Google Scholar] [CrossRef]
- Keenan, T.W.; Morré, D.J. Phospholipid class and fatty acid composition of golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry 1970, 9, 19–25. [Google Scholar] [CrossRef]
- Ingólfsson, H.I.; Melo, M.N.; van Eerden, F.J.; Arnarez, C.; Lopez, C.A.; Wassenaar, T.A.; Periole, X.; de Vries, A.H.; Tieleman, D.P.; Marrink, S.J. Lipid Organization of the Plasma Membrane. J. Am. Chem. Soc. 2014, 136, 14554–14559. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [PubMed]
- Duda, M.; Kawula, K.; Pawlak, A.; Sarna, T.; Wisniewska-Becker, A. EPR Studies on the Properties of Model Photoreceptor Membranes Made of Natural and Synthetic Lipids. Cell Biochem. Biophys. 2017, 75, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, K.M. Functional materials based on molecules with hydrogen-bonding ability: Applications to drug co-crystals and polymer complexes. R. Soc. Open Sci. 2018, 5, 180564. [Google Scholar] [CrossRef]
- Ohto, T.; Backus, E.H.G.; Hsieh, C.-S.; Sulpizi, M.; Bonn, M.; Nagata, Y. Lipid Carbonyl Groups Terminate the Hydrogen Bond Network of Membrane-Bound Water. J. Phys. Chem. Lett. 2015, 6, 4499–4503. [Google Scholar] [CrossRef] [PubMed]
- Dreier, L.B.; Bonn, M.; Backus, E.H.G. Hydration and Orientation of Carbonyl Groups in Oppositely Charged Lipid Monolayers on Water. J. Phys. Chem. B 2019, 123, 1085–1089. [Google Scholar] [CrossRef]
- Nakanishi, K.; Fishkin, N.; Berova, N. Chapter 19-CD and Visual Science. In Progress in Biological Chirality; Pályi, G., Zucchi, C., Caglioti, L., Eds.; Elsevier Science Ltd.: Oxford, UK, 2004; pp. 237–251. [Google Scholar]
- Send, R.; Sundholm, D. The molecular structure of a curl-shaped retinal isomer. J. Mol. Model. 2008, 14, 717–726. [Google Scholar] [CrossRef]
- Pályi, G.; Zucchi, C.; Caglioti, L. Chapter 2-Dimensions of Biological Homochirality. In Advances in BioChirality; Pályi, G., Zucchi, C., Caglioti, L., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1999; pp. 3–12. [Google Scholar]
- Albert, A.D.; Boesze-Battaglia, K. The role of cholesterol in rod outer segment membranes. Prog. Lipid Res. 2005, 44, 99–124. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Litman, B.J. Effect of Cholesterol on Molecular Order and Dynamics in Highly Polyunsaturated Phospholipid Bilayers. Biophys. J. 1998, 75, 896–908. [Google Scholar] [CrossRef]
- Martin, R.E.; Elliott, M.H.; Brush, R.S.; Anderson, R.E. Detailed Characterization of the Lipid Composition of Detergent-Resistant Membranes from Photoreceptor Rod Outer Segment Membranes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1147–1154. [Google Scholar] [CrossRef]
- London, E.; Brown, D.A. Insolubility of lipids in Triton X-100: Physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Et Biophys. Acta (BBA) Biomembr. 2000, 1508, 182–195. [Google Scholar] [CrossRef]
- Wiśniewska, A.; Subczynski, W.K. Accumulation of macular xanthophylls in unsaturated membrane domains. Free. Radic. Biol. Med. 2006, 10, 1820–1826. [Google Scholar] [CrossRef]
- Gandhavadi, M.; Allende, D.; Vidal, A.; Simon, S.A.; McIntosh, T.J. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 2002, 82, 1469–1482. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bretillon, L.; Thuret, G.; Gregoire, S.; Acar, N.; Joffre, C.; Bron, A.M.; Gain, P.; Creuzot-Garcher, C.P. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp. Eye Res. 2008, 87, 521–528. [Google Scholar] [CrossRef]
- Ueda, K.; Zhao, J.; Kim, H.J.; Sparrow, J.R. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 6904–6909. [Google Scholar] [CrossRef]
- Tarapcsák, S.; Szalóki, G.; Telbisz, Á.; Gyöngy, Z.; Matúz, K.; Csősz, É.; Nagy, P.; Holb, I.J.; Rühl, R.; Nagy, L.; et al. Interactions of retinoids with the ABC transporters P-glycoprotein and Breast Cancer Resistance Protein. Sci. Rep. 2017, 7, 41376. [Google Scholar] [CrossRef]
- Nossoni, Z.; Assar, Z.; Yapici, I.; Nosrati, M.; Wang, W.; Berbasova, T.; Vasileiou, C.; Borhan, B.; Geiger, J. Structures of holo wild-type human cellular retinol-binding protein II (hCRBPII) bound to retinol and retinal. Acta Crystallogr. Sect. D 2014, 70, 3226–3232. [Google Scholar] [CrossRef]
- Falck, E.; Patra, M.; Karttunen, M.; Hyvönen, M.T.; Vattulainen, I. Impact of cholesterol on voids in phospholipid membranes. J. Chem. Phys. 2004, 121, 12676–12689. [Google Scholar] [CrossRef]
- Foley, S.; Miller, E.; Braziel, S.; Lee, S. Molecular organization in mixed SOPC and SDPC model membranes: Water permeability studies of polyunsaturated lipid bilayers. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183365. [Google Scholar] [CrossRef]
- Bouffioux, O.; Berquand, A.; Eeman, M.; Paquot, M.; Dufrêne, Y.F.; Brasseur, R.; Deleu, M. Molecular organization of surfactin–phospholipid monolayers: Effect of phospholipid chain length and polar head. Biochim. Et Biophys. Acta (BBA) Biomembr. 2007, 1768, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Onuki, Y.; Morishita, M.; Chiba, Y.; Tokiwa, S.; Takayama, K. Docosahexaenoic acid and eicosapentaenoic acid induce changes in the physical properties of a lipid bilayer model membrane. Chem. Pharm. Bull. 2006, 54, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Allen, C. Differential Rhodopsin Regeneration in Photoreceptor Membranes is Correlated with Variations in Membrane Properties. Biosci. Rep. 1998, 18, 29–38. [Google Scholar] [CrossRef]
- Nickell, S.; Park, P.S.; Baumeister, W.; Palczewski, K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J. Cell Biol. 2007, 177, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Quazi, F.; Molday, R.S. ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal. Proc. Natl. Acad. Sci. USA 2014, 111, 5024–5029. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Brown, D.A.; London, E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 1997, 36, 10944–10953. [Google Scholar] [CrossRef]














| Plasma Membrane | Young Disc Membrane | Old Disc Membrane | |
|---|---|---|---|
| PC:PE ratio PUFA content | PC:PE, 5:1 PUFA 5 mol% | PC:PE, 1:1 PUFA 35 mol% | PC:PE, 1:1 PUFA 35 mol% |
| DMPC | 2 mM | 1 mM | 1 mM |
| POPC | 3 mM | 1.5 mM | 2.75 mM |
| (16:0)(22:6)PC | - | 1 mM | 1. mM |
| (16:0)(22:6)PE | 0.5 mM | 2.5 mM | 2.5 mM |
| POPE | 0.5 mM | 1 mM | 2.25 mM |
| Cholesterol | 4 mM | 3 mM | 0.5 mM |
| n-PC | 0.1 mM | 0.1 mM | 0.1 mM |
| Retinoid | 0 mM (control) or 1 mM | 0 mM (control) or 1 mM | 0 mM (control) or 1 mM |
| POPC-WATER | POPC Op-WATER | POPC Oc-WATER | RET-WATER | |
|---|---|---|---|---|
| REF | 7.07 ± 0.1 | 5.27 ± 0.07 | 1.8 ± 0.06 | ——— |
| 11-cis RAL | 7.14 ± 0.1 | 5.32 ± 0.07 | 1.83 ± 0.06 | 0.41 ± 0.15 |
| All-trans RAL | 7.13 ± 0.1 | 5.31 ± 0.07 | 1.82 ± 0.06 | 0.63 ± 0.16 |
| All-trans RAL DIMER | 7.15 ± 0.1 | 5.32 ± 0.07 | 1.83 ± 0.06 | 0.41 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzin, S.; Wiśniewska-Becker, A.; Markiewicz, M.; Bętkowski, S.; Furso, J.; Waresiak, J.; Grolik, J.; Sarna, T.; Pawlak, A.M. Structural Impact of Selected Retinoids on Model Photoreceptor Membranes. Membranes 2023, 13, 575. https://doi.org/10.3390/membranes13060575
Radzin S, Wiśniewska-Becker A, Markiewicz M, Bętkowski S, Furso J, Waresiak J, Grolik J, Sarna T, Pawlak AM. Structural Impact of Selected Retinoids on Model Photoreceptor Membranes. Membranes. 2023; 13(6):575. https://doi.org/10.3390/membranes13060575
Chicago/Turabian StyleRadzin, Szymon, Anna Wiśniewska-Becker, Michał Markiewicz, Sebastian Bętkowski, Justyna Furso, Joanna Waresiak, Jarosław Grolik, Tadeusz Sarna, and Anna M. Pawlak. 2023. "Structural Impact of Selected Retinoids on Model Photoreceptor Membranes" Membranes 13, no. 6: 575. https://doi.org/10.3390/membranes13060575
APA StyleRadzin, S., Wiśniewska-Becker, A., Markiewicz, M., Bętkowski, S., Furso, J., Waresiak, J., Grolik, J., Sarna, T., & Pawlak, A. M. (2023). Structural Impact of Selected Retinoids on Model Photoreceptor Membranes. Membranes, 13(6), 575. https://doi.org/10.3390/membranes13060575