Green Synthesis of Cation Exchange Membranes: A Review
Abstract
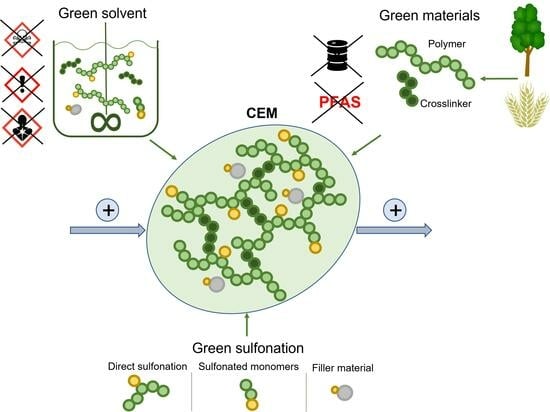
:1. Introduction
2. Green vs. Sustainable Chemistry
3. Greenness and Sustainability of Perfluorinated Membranes
4. Green Sulfonation
5. Green Materials
6. Green Solvents
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morens, D.M.; Fauci, A.S. Emerging Pandemic Diseases: How We Got to COVID-19. Cell 2020, 182, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- von Schneidemesser, E.; Monks, P.S.; Allan, J.D.; Bruhwiler, L.; Forster, P.; Fowler, D.; Lauer, A.; Morgan, W.T.; Paasonen, P.; Mattia, R.; et al. Chemistry and the Linkages between Air Quality and Climate Change. Chem. Rev. 2015, 115, 3856–3897. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.G.; Vangipuram, B.; Dalin, C.; Erfani, T. Water Quality and Pollution Trading: A Sustainable Solution for Future Food Production. ACS EST Eng. 2023, 3, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, T.; Hague, C.; Cole, D.; Thomas, E.; Duarte-Davidson, R. Global event-based surveillance of chemical incidents. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Minotti, A.; Fontananova, E.; Longo, M.; Jansen, J.; Figoli, A. Green H2 Production by Water Electrolysis Using Cation Exchange Membrane: Insights on Activation and Ohmic Polarization Phenomena. Membranes 2021, 12, 15. [Google Scholar] [CrossRef]
- Chabi, S.; Papadantonakis, K.M.; Lewis, N.S.; Freund, M.S. Membranes for artificial photosynthesis. Energy Environ. Sci. 2017, 10, 1320–1338. [Google Scholar] [CrossRef]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Sedighi, M.; Usefi, M.M.B.; Ismail, A.F.; Ghasemi, M. Environmental sustainability and ions removal through electrodialysis desalination: Operating conditions and process parameters. Desalination 2023, 549, 116319. [Google Scholar] [CrossRef]
- Juve, J.-M.A.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A comprehensive review on the synthesis and applications of ion exchange membranes. Chemosphere 2021, 282, 130817. [Google Scholar] [CrossRef] [PubMed]
- Grot, W. Manufacture. In Fluorinated Ionomers; Elsevier: Amsterdam, The Netherlands, 2011; pp. 11–48. [Google Scholar] [CrossRef]
- Okazoe, T.; Watanabe, K.; Itoh, M.; Shirakawa, D.; Kawahara, K.; Tatematsu, S. Synthesis of perfluorinated carboxylic acid membrane monomers by utilizing liquid-phase direct fluorination. J. Fluor. Chem. 2005, 126, 519–525. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Perfluorinated ion-exchange membranes. Polym. Sci. Ser. A 2013, 55, 674–698. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Compounds, E.L.L.P. Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Houde, M.; De Silva, A.O.; Muir, D.C.G.; Letcher, R.J. Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environ. Sci. Technol. 2011, 45, 7962–7973. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, S.A.N.; Vatanpour, V.; Koyuncu, I. Green solvents in polymeric membrane fabrication: A review. Sep. Purif. Technol. 2022, 298, 121691. [Google Scholar] [CrossRef]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef]
- Muhmed, S.A.; Nor, N.A.M.; Jaafar, J.; Ismail, A.S.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusolf, N. Emerging chitosan and cellulose green materials for ion exchange membrane fuel cell: A review. Energy Ecol. Environ. 2020, 5, 85–107. [Google Scholar] [CrossRef]
- Anastas, P.; John, W. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Environmental Protection Agency. Green Chemistry. Available online: https://www.epa.gov/greenchemistry (accessed on 5 November 2023).
- Lewandowski, T.A. Green Chemistry. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 798–799. [Google Scholar] [CrossRef]
- Hogue, C. 2023. Available online: https://cen.acs.org/environment/green-chemistry/Differentiating-between-green-chemistry-sustainable/97/web/2019/07 (accessed on 5 November 2023).
- Kümmerer, K. Sustainable Chemistry: A Future Guiding Principle. Angew. Chem. Int. Ed. 2017, 56, 16420–16421. [Google Scholar] [CrossRef]
- Anta, M.E.; Alonso, C.G.; Tagliavini, E.; Sainz, D. Sustainable chemistry. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Hyseni, V. Green Sustainability. PECB Website. 2021. Available online: https://pecb.com/article/green-sustainability#:~:text=Going%20green% (accessed on 16 October 2023).
- Halpaap, A. Green and Sustainable Chemistry: Framework Manual. Available online: https://www.unep.org/resources/toolkits-manuals-and-guides/green-and-sustainable-chemistry-framework-manual (accessed on 17 October 2023).
- Gauthier, A.M.; Fung, M.; Panko, J.; Kingsbury, T.; Perez, A.L.; Hitchcock, K.; Ferracini, T.; Sahmel, J.; Banducci, A.; Jacobsen, M.; et al. Chemical assessment state of the science: Evaluation of 32 decision-support tools used to screen and prioritize chemicals. Integr. Environ. Assess. Manag. 2015, 11, 242–255. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes. Org. Process Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Zhou, J.; Baumann, K.; Surratt, J.D.; Turpin, B.J. Legacy and emerging airborne per- and polyfluoroalkyl substances (PFAS) collected on PM 2.5 filters in close proximity to a fluoropolymer manufacturing facility. Environ. Sci. Process. Impacts 2022, 24, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Christiansen, E. A case study of a TFE explosion in a PTFE manufacturing facility. Process Saf. Prog. 2007, 26, 77–82. [Google Scholar] [CrossRef]
- Demeneix, B.A. How fossil fuel-derived pesticides and plastics harm health, biodiversity, and the climate. Lancet Diabetes Endocrinol. 2020, 8, 462–464. [Google Scholar] [CrossRef]
- Parekh, A. Recent developments of proton exchange membranes for PEMFC: A review. Front. Energy Res. 2022, 10, 956132. [Google Scholar] [CrossRef]
- Kim, Y.S.; Pivovar, B.S. Polymer Electrolyte Membranes for Direct Methanol Fuel Cells. Adv. Fuel Cells 2007, 1, 187–234. [Google Scholar] [CrossRef]
- Resnick, P. Une Courte Histoire du Nafion®. l’Actualité Chimique. 2006. n° 301–302. Available online: https://new.societechimiquedefrance.fr/numero/une-courte-histoire-du-nafion-p144-n301-302/ (accessed on 18 October 2023).
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Kim, Y.S.; Welch, C.F.; Hjelm, R.P.; Mack, N.H.; Labouriau, A.; Orler, E.B. Origin of Toughness in Dispersion-Cast Nafion Membranes. Macromolecules 2015, 48, 2161–2172. [Google Scholar] [CrossRef]
- Grot, W. CF=CFCF 2 CF SO F and Dervatives and Polymers Thereof. U.S. Patent 3718627, 27 February 1973. [Google Scholar]
- Maiyalagan, T.; Pasupathi, S. Components for PEM Fuel Cells: An Overview. Mater. Sci. Forum 2010, 657, 143–189. [Google Scholar] [CrossRef]
- Haubold, H.-G.; Vad, T.; Jungbluth, H.; Hiller, P. Nano structure of NAFION: A SAXS study. Electrochim. Acta 2001, 46, 1559–1563. [Google Scholar] [CrossRef]
- James, C.D.; Franklin, G.W. Fluorocarbon Vinyl ether Polymers. U.S. Patent 3282875, 1 November 1966. Available online: https://patentimages.storage.googleapis.com/a0/7f/dc/588ef81742e519/US3282875.pdf (accessed on 19 October 2023).
- Ervin, P.R.; Dickson, N.W. Fluorocarbon ethers Containing Sulfonyl Groups. U.S. Patent 3301893, 31 January 1967. [Google Scholar]
- England, D.C.; Oak, H.; Del. A-Sulfopolyfluoromonocarboxylic Acds and Dervatives Hydrolyzable Thereto. U.S. Patent 597321, 16 September 1958. Available online: https://patentimages.storage.googleapis.com/25/df/03/533c23114c92c9/US2852554.pdf (accessed on 19 October 2023).
- Siegemund, G.; Schwertfeger, W.; Feiring, A.; Smart, B.; Behr, F.; Vogel, H.; McKustick, B.; Kirsch, P. Fluorine Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 1–56. [Google Scholar] [CrossRef]
- U.S. Department of Labor. In OSHA News Release-Region 4. June 2022. Available online: https://www.osha.gov/news/newsreleases/region4/01052022 (accessed on 16 November 2023).
- ECHA. Per- and Polyfluoroalkyl Substances (PFAS). Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 20 October 2023).
- Feng, M.; Qu, R.; Wei, Z.; Wang, L.; Sun, P.; Wang, Z. Characterization of the thermolysis products of Nafion membrane: A potential source of perfluorinated compounds in the environment. Sci. Rep. 2015, 5, 9859. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yao, Y.; Zhao, Z.; Wang, Y.; Wang, Q.; Ren, C.; Wang, B.; Sun, H.; Alder, C.A.; Kannan, K. Multimedia Distribution and Transfer of Per- and Polyfluoroalkyl Substances (PFASs) Surrounding Two Fluorochemical Manufacturing Facilities in Fuxin, China. Environ. Sci. Technol. 2018, 52, 8263–8271. [Google Scholar] [CrossRef]
- Hydrogen Europe. Hydrogen Europe Position Paper on PFAS. 2023. Available online: https://hydrogeneurope.eu/wp-content/uploads/2023/02/Hydrogen-Europe-position-paper-on-PFAS-ban_v12_FINAL.pdf (accessed on 20 October 2023).
- Okazoe, T. Perfluorination with F2. In Fluorination; Springer: Singapore, 2020; pp. 513–521. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Rynkowska, E. Advancements in proton exchange membranes for high-performance high-temperature proton exchange membrane fuel cells (HT-PEMFC). Rev. Chem. Eng. 2022, 38, 327–346. [Google Scholar] [CrossRef]
- Li, C.; Song, K.; Hao, C.; Liang, W.; Li, X.; Zhang, W.; Wang, Y.; Song, Y. Fabrication of S-PBI cation exchange membrane with excellent anti-fouling property for enhanced performance in electrodialysis. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130910. [Google Scholar] [CrossRef]
- Shi, H. Sulfonation mechanism of benzene with SO3 in sulfuric acid or oleum or aprotic solvent: Obeying the transition state theory via a trimolecular electrophilic substitution clarified by density functional theory calculation. Comput. Theor. Chem. 2017, 1112, 111–122. [Google Scholar] [CrossRef]
- Galabov, B.; Nalbantova, D.; Schleyer, P.V.R.; Schaefer, H.F. Electrophilic Aromatic Substitution: New Insights into an Old Class of Reactions. Acc. Chem. Res. 2016, 49, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Koleva, G.; Galabov, B.; Kong, J.; Schaefer, H.F., III; Schleyer, P.V.R. Electrophilic Aromatic Sulfonation with SO 3: Concerted or Classic S E Ar Mechanism? J. Am. Chem. Soc. 2011, 133, 19094–19101. [Google Scholar] [CrossRef] [PubMed]
- Khomein, P.; Ketelaars, W.; Lap, T.; Liu, G. Sulfonated aromatic polymer as a future proton exchange membrane: A review of sulfonation and crosslinking methods. Renew. Sustain. Energy Rev. 2021, 137, 110471. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Li, H.; Wang, W. Risk analysis of gaseous SO3 sulfonation reactor system based on HAZOP-LOPA. In Proceedings of the 2020 IEEE 3rd International Conference of Safe Production and Informatization (IICSPI), Chongqing, China, 28–30 November 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 361–365. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.; Meng, W.; Yuan, H.; Ma, W.; Sun, X.; Xu, J.; Tan, B.; Li, P. Continuous sulfonation of hexadecylbenzene in a microreactor. Green Process. Synth. 2021, 10, 219–229. [Google Scholar] [CrossRef]
- Kucera, F.; Jancar, J. Sulfonation of solid polystyrene using gaseous sulfur trioxide. Polym. Eng. Sci. 2009, 49, 1839–1845. [Google Scholar] [CrossRef]
- Wang, R.; Han, H.H.; Liu, F.Q.; Jia, S.X.; Xiang, T.Q.; Huo, H.; Zhou, J.J.; Li, L. Sulfonated poly(vinyl alcohol) as an artificial solid electrolyte interfacial layer for Li metal anode. Electrochim. Acta 2022, 406, 139840. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Safety Data Sheet Vinylsulfonic Acid Sodium Salt Solution (25%). 2023. Available online: https://www.sigmaaldrich.com/BE/en/sds/aldrich/278416 (accessed on 8 November 2023).
- TCI America. Safety Data Sheet Vinylsulfonic Acid TCl America. 2018. Available online: https://www.tcichemicals.com/US/en/p/V0134#docomentsSectionPDP (accessed on 8 November 2023).
- Breslow, D.S.; Hough, R.R. The Synthesis of Sodium Ethylenesulfonate from Ethylene. J. Am. Chem. Soc. 1957, 79, 5000–5002. [Google Scholar] [CrossRef]
- Schenk, W.; Dahlinger, R.; Stockburger, D. Manufacture of Vinyl Sulfonates and Vinylsulfonic Acid from Carbyl Sulfate. U.S. Patent 3872165A, 18 March 1975. [Google Scholar]
- Thakur, A.K.; Pandey, R.P.; Shahi, V.K. Preparation characterization and thermal degradation studies of bi-functional cation-exchange membranes. Desalination 2015, 367, 206–215. [Google Scholar] [CrossRef]
- Miller, L.; Donald, M. Preparation of Acrylamidoalkanesulfonic Acids. U.S. Patent 3506707A, 14 April 1970. Available online: https://worldwide.espacenet.com/patent/search/family/024820233/publication/US3506707A?q=pn%3DUS3506707 (accessed on 8 November 2023).
- Deboli, F.; Van der Bruggen, B.; Donten, M.L. A novel concept of hierarchical cation exchange membrane fabricated from commodity precursors through an easily scalable process. J. Membr. Sci. 2021, 636, 119594. [Google Scholar] [CrossRef]
- Ngo, H.L.; Nguyen, N.T.; Ho, T.T.N.; Pham, H.V.; Le, T.N.; Tran, T.N.; Huynh, L.T.N.; Pham, T.N.; Nguyen, T.T.; Nguyen, T.H.; et al. The performance of MCDI: The effect of sulfosuccinic acid ratio in the PVA-based cation exchange membrane. Ionics 2022, 28, 4369–4380. [Google Scholar] [CrossRef]
- Guidechem. SDS Sulfosuccinic Acid. 2017. Available online: https://www.guidechem.com/msds/5138-18-1.html (accessed on 8 November 2023).
- Wibel, R.; Knoll, P.; Le-Vinh, B.; Kali, G.; Bernkop, A.-. Schnürch. Synthesis and evaluation of sulfosuccinate-based surfactants as counterions for hydrophobic ion pairing. Acta Biomater. 2022, 144, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Kisielewski, J.; Robertson, D. Process for production of sodium bisulfite. U.S. Patent 20100008845A1, 26 July 2011. [Google Scholar]
- Airgas. Safety Data Sheet SO2 Airgas. Available online: https://www.airgas.com/msds/001047.pdf (accessed on 8 November 2023).
- Deboli, F.; Van der Bruggen, B.; Donten, M.L. A versatile chemistry platform for the fabrication of cost-effective hierarchical cation and anion exchange membranes. Desalination 2022, 535, 115794. [Google Scholar] [CrossRef]
- Firganek, D.; Donten, M.L.; Van, B. der Bruggen. Impact of Formulation of Photocurable Precursor Mixtures on the Performance and Dimensional Stability of Hierarchical Cation Exchange Membranes. Ind. Eng. Chem. Res. 2023, 62, 15928–15939. [Google Scholar] [CrossRef]
- Niederhauser, W.; Edward, B.; Owings, F. Method for Preparing Salts of Sulfoalkyl Methacrylates. U.S. Patent 2964557A, 13 March 1958. Available online: https://patents.google.com/patent/US2964557 (accessed on 8 November 2023).
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Deng, S.; Takeuchi, N.; Hieda, J.; Takahashi, K.; Tachibana, K.; Li, O.L. Investigation of the sulfonation mechanism by gas–liquid interfacial plasma under atmospheric pressure conditions. J. Phys. D Appl. Phys. 2022, 55, 345205. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Madaeni, S.S.; Khodabakhshi, A.R. Preparation and characterization of PC/SBR heterogeneous cation exchange membrane filled with carbon nano-tubes. J. Memb. Sci. 2010, 362, 550–559. [Google Scholar] [CrossRef]
- Shoparwe, N.F.; Ahmad, A.L.; Ahmad, N.A.; Shafie, M.; Fabrication, Z.M.H. Characterisation and Electrochemical Properties of Heterogeneous Multiwalled Carbon Nanotubes Cation Exchange Membranes (MWCNT-CEMs). J. Phys. Sci. 2018, 29 (Suppl. 1), 41–48. [Google Scholar] [CrossRef]
- Chien, H.-C.; Tsai, L.-D.; Huang, C.-P.; Kang, C.; Lin, J.-N.; Chang, F.-C. Sulfonated graphene oxide/Nafion composite membranes for high-performance direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 13792–13801. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on Sulfonated Poly (Ether Ether Ketone) membrane for direct methanol fuel cells. J. Memb. Sci. 2013, 425–426, 11–22. [Google Scholar] [CrossRef]
- Gahlot, S.; Sharma, P.P.; Kulshrestha, V. Dramatic Improvement in Ionic Conductivity and Water Desalination Efficiency of SGO Composite Membranes. Sep. Sci. Technol. 2015, 50, 446–453. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Manthiram, A. Sulfonated poly(ether ether ketone) membranes with sulfonated graphene oxide fillers for direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 5875–5884. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsu, C.-Y.; Su, Y.-H.; Lai, J.-Y. Chitosan−Silica Complex Membranes from Sulfonic Acid Functionalized Silica Nanoparticles for Pervaporation Dehydration of Ethanol−Water Solutions. Biomacromolecules 2005, 6, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Suryani; Liu, Y.L. Preparation and properties of nanocomposite membranes of polybenzimidazole/sulfonated silica nanoparticles for proton exchange membranes. J. Memb. Sci. 2009, 332, 121–128. [Google Scholar] [CrossRef]
- Jun, Y.; Zarrin, H.; Fowler, M.; Chen, Z. Functionalized titania nanotube composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6073–6081. [Google Scholar] [CrossRef]
- Yun, S.; Im, H.; Heo, Y.; Kim, J. Crosslinked sulfonated poly(vinyl alcohol)/sulfonated multi-walled carbon nanotubes nanocomposite membranes for direct methanol fuel cells. J. Memb. Sci. 2011, 380, 208–215. [Google Scholar] [CrossRef]
- Fan, H.; Huang, Y.; Yip, N.Y. Advancing the conductivity-permselectivity tradeoff of electrodialysis ion-exchange membranes with sulfonated CNT nanocomposites. J. Memb. Sci. 2020, 610, 118259. [Google Scholar] [CrossRef]
- Qin, L.; Ishizaki, T.; Takeuchi, N.; Takahashi, K.; Kim, K.H.; Li, O.L. Green Sulfonation of Carbon Catalysts via Gas–Liquid Interfacial Plasma for Cellulose Hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 5837–5846. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.-M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Seop, H. Kim. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [PubMed]
- Chowdury, M.S.K.; Cho, Y.J.; Park, S.B.; Park, Y. Enhanced Proton Conductivity of (3-mercaptopropyl)trimethoxysilane—Grafted Graphene Oxide Membranes for Hydrogen Fuel Cells. J. Electrochem. Soc. 2021, 168, 124502. [Google Scholar] [CrossRef]
- Mosa, J.; Durán, A.; Aparicio, M. Sulfonic acid-functionalized hybrid organic–inorganic proton exchange membranes synthesized by sol–gel using 3-mercaptopropyl trimethoxysilane (MPTMS). J. Power Sources 2015, 297, 208–216. [Google Scholar] [CrossRef]
- Kajau, A.; Motsa, M.; Mamba, B.B.; Mahlangu, O. Leaching of CuO Nanoparticles from PES Ultrafiltration Membranes. ACS Omega 2021, 6, 31797–31809. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes By Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef]
- Hanif, Z.; Khan, Z.A.; Siddiqui, M.F.; Tariq, M.Z.; Park, S.; Park, S.J. Tannic acid-mediated rapid layer-by-layer deposited non-leaching silver nanoparticles hybridized cellulose membranes for point-of-use water disinfection. Carbohydr. Polym. 2020, 231, 115746. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Paziresh, S.; Zinadini, S.; Zinatizadeh, A.A.; Koyuncu, I. Polylactic acid in the fabrication of separation membranes: A review. Sep. Purif. Technol. 2022, 296, 121433. [Google Scholar] [CrossRef]
- Mehrabian, M.; Kargari, A. Bio-based nonporous membranes: Evolution and benchmarking review. J. Ind. Eng. Chem. 2023, 124, 17–39. [Google Scholar] [CrossRef]
- Binsu, V.V.; Nagarale, R.K.; Shahi, V.K.; Ghosh, P.K. Studies on N-methylene phosphonic chitosan/poly(vinyl alcohol) composite proton-exchange membrane. React. Funct. Polym. 2006, 66, 1619–1629. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Functionalized Organic−Inorganic Nanostructured N-p-Carboxy Benzyl Chitosan−Silica−PVA Hybrid Polyelectrolyte Complex as Proton Exchange Membrane for DMFC Applications. J. Phys. Chem. B 2008, 112, 15678–15690. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, C.; Wang, J.; Liu, X.; Liu, H.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C.; Wen, S. Chitosan/silica coated carbon nanotubes composite proton exchange membranes for fuel cell applications. Carbohydr. Polym. 2016, 136, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Namboodiri, M.M.T.; Pakshirajan, K. Sustainable and green approach of chitosan production from Penicillium citrinum biomass using industrial wastewater as a cheap substrate. J. Environ. Manag. 2019, 240, 431–440. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef]
- Chopra, L. Extraction of cellulosic fibers from the natural resources: A short review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Vadanan, S.V.; Basu, A.; Lim, S. Bacterial cellulose production, functionalization, and development of hybrid materials using synthetic biology. Polym. J. 2022, 54, 481–492. [Google Scholar] [CrossRef]
- Yue, L.; Xie, Y.; Zheng, Y.; He, W.; Guo, S.; Sun, Y.; Zhang, T.; Liu, S. Sulfonated bacterial cellulose/polyaniline composite membrane for use as gel polymer electrolyte. Compos. Sci. Technol. 2017, 145, 122–131. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Elmageed, M.H.A.; Omer, A.M.; Tamer, T.M.; Yossuf, M.E.; Khalifa, R.E. Novel Proton Exchange Membranes Based on Sulfonated Cellulose Acetate for Fuel Cell Applications: Preparation and Characterization. Int. J. Electrochem. Sci. 2016, 11, 10150–10171. [Google Scholar] [CrossRef]
- Miller, B.G. Emissions Control Strategies for Power Plants. In Clean Coal Engineering Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 375–481. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA based biocomposites for sustainable products: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorksTM polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Wang, M.-Y.; Suen, S.-Y. Eco-friendly polylactic acid/rice husk ash mixed matrix membrane for efficient purification of lysozyme from chicken egg white. J. Taiwan Inst. Chem. Eng. 2020, 111, 11–23. [Google Scholar] [CrossRef]
- Xiong, Y.; Cui, X.; Wang, D.; Wang, Y.; Lou, Z.; Shan, W.; Fan, Y. Diethanolamine functionalized rice husk for highly efficient recovery of gallium(III) from solution and a mechanism study. Mater. Sci. Eng. C 2019, 99, 1115–1122. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Nagar, H.; Sumana, C.; Rao, V.V.B.; Sridhar, S. Performance evaluation of sodium alginate–Pebax polyion complex membranes for application in direct methanol fuel cells. J. Appl. Polym. Sci. 2017, 134, 44485. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Udo, T.; Mummaleti, G.; Mohan, A.; Singh, R.K.; Kong, F. Current and emerging applications of carrageenan in the food industry. Food Res. Int. 2023, 173, 113369. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Falqué, E.; Domínguez, H.; Torres, M.D. Green Extraction of Carrageenans from Mastocarpus stellatus. Polymers 2022, 14, 554. [Google Scholar] [CrossRef]
- Pahnavar, Z.; Ghaemy, M.; Naji, L.; Hasantabar, V. Self-extinguished and flexible cation exchange membranes based on modified K-Carrageenan/PVA double network hydrogels for electrochemical applications. Int. J. Biol. Macromol. 2023, 231, 123253. [Google Scholar] [CrossRef]
- Gouda, M.H.; Tamer, T.M.; Konsowa, A.H.; Farag, H.A.; Mohy Eldin, M.S. Organic-Inorganic Novel Green Cation Exchange Membranes for Direct Methanol Fuel Cells. Energies 2021, 14, 4686. [Google Scholar] [CrossRef]
- Liew, J.W.Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Wan Daud, W.R. Synthesis and characterization of modified κ-carrageenan for enhanced proton conductivity as polymer electrolyte membrane. PLoS ONE 2017, 12, e0185313. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Farag, H.A.; Tamer, T.M.; Konsowa, A.H.; Gouda, M.H. Development of novel iota carrageenan-g-polyvinyl alcohol polyelectrolyte membranes for direct methanol fuel cell application. Polym. Bull. 2020, 77, 4895–4916. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Where do poly(vinyl alcohol) based membranes stand in relation to Nafion® for direct methanol fuel cell applications? J. Power Sources 2012, 216, 48–66. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous Polyvinyl Alcohol Membranes: Preparation Methods and Applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Regina, S.; Poerio, T.; Mazzei, R.; Sabia, C.; Iseppi, R.; Giorno, L. Pectin as a non-toxic crosslinker for durable and water-resistant biopolymer-based membranes with improved mechanical and functional properties. Eur. Polym. J. 2022, 172, 111193. [Google Scholar] [CrossRef]
- Gautam, L.; Warkar, S.G.; Ahmad, S.I.; Kant, R.; Jain, M. A review on carboxylic acid cross-linked polyvinyl alcohol: Properties and applications. Polym. Eng. Sci. 2022, 62, 225–246. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, S.-B.; Han, N.-W. Kinetics of crosslinking reaction of PVA membrane with glutaraldehyde. Korean J. Chem. Eng. 1994, 11, 41–47. [Google Scholar] [CrossRef]
- Nascimento, F.C.D.; Vianna de Aguiar, L.C.; Costa, L.A.T.; Fernandes, M.T.; Marassi, R.J.; Gomes, A.S.; Adilson de Castro, J. Formulation and characterization of crosslinked polyvinyl alcohol (PVA) membranes: Effects of the crosslinking agents. Polym. Bull. 2021, 78, 917–929. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Ezati, P.; Yang, D.-P.; Rhim, J.-W. Tannic acid: A green crosslinker for biopolymer-based food packaging films. Trends Food Sci. Technol. 2023, 136, 11–23. [Google Scholar] [CrossRef]
- Moghaddam, S.Y.Z.; Biazar, E.; Esmaeili, J.; Kheilnezhad, B.; Goleij, F.; Heidari, S. Tannic Acid as a Green Cross-linker for Biomaterial Applications. Mini-Rev. Med. Chem. 2023, 23, 1320–1340. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Cao, R. Robust antifouling anion exchange membranes modified by graphene oxide (GO)-enhanced Co-deposition of tannic acid and polyethyleneimine. J. Memb. Sci. 2021, 625, 119111. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Zhang, Y.; Zhang, Y.; Hong, G.; Moutloali, R.M.; Mamba, B.B.; Li, F.; Ma, J.; Shao, L. Mussel-inspired tannic acid/polyethyleneimine assembling positively-charged membranes with excellent cation permselectivity. Sci. Total Environ. 2022, 817, 153051. [Google Scholar] [CrossRef]
- Chui, C.; Odeleye, A.; Nguyen, L.; Kasoju, N.; Soliman, E.; Ye, H. Electrosprayed genipin cross-linked alginate–chitosan microcarriers for ex vivo expansion of mesenchymal stem cells. J. Biomed. Mater. Res. A 2019, 107, 122–133. [Google Scholar] [CrossRef]
- Gorgieva, S.; Osmić, A.; Genorio, B.; Hacker, V.; Wolf, S.; Hribernik, S. The efficiency of chitosan-graphene oxide composite membranes modified with genipin in fuel cell application. E3S Web Conf. 2022, 334, 04002. [Google Scholar] [CrossRef]
- Du, J.R.; Hsu, L.H.; Xiao, E.S.; Guo, X.; Zhang, Y.; Feng, X. Using genipin as a ‘green’ crosslinker to fabricate chitosan membranes for pervaporative dehydration of isopropanol. Sep. Purif. Technol. 2020, 244, 116843. [Google Scholar] [CrossRef]
- Kim, D.; Salazar, O.R.; Nunes, S.P. Membrane manufacture for peptide separation. Green Chem. 2016, 18, 5151–5159. [Google Scholar] [CrossRef]
- Jiang, X.; Yong, W.F.; Gao, J.; Shao, D.-D.; Sun, S.-P. Understanding the role of substrates on thin film composite membranes: A green solvent approach with TamiSolve® NxG. J. Memb. Sci. 2021, 635, 119530. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Hofstetter, T.B.; Capello, C.; Hungerbühler, K. Environmentally Preferable Treatment Options for Industrial Waste Solvent Management. Process Saf. Environ. Prot. 2003, 81, 189–202. [Google Scholar] [CrossRef]
- Amelio, A.; Genduso, G.; Vreysen, S.; Luis, P.; Van, B.d.B. Guidelines based on life cycle assessment for solvent selection during the process design and evaluation of treatment alternatives. Green Chem. 2014, 16, 3045–3063. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Simone, S.; Di Nicolò, D.; Li, X.-M.; He, T.; Tornaghi, S.; Drioli, E. Towards non-toxic solvents for membrane preparation: A review. Green Chem. 2014, 16, 4034. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Criscuoli, A.; Figoli, A. TamiSolve® NxG as novel solvent for polymeric membrane preparation. J. Memb. Sci. 2017, 542, 418–429. [Google Scholar] [CrossRef]
- Ma, C.-H.; Yu, T.L.; Lin, H.-L.; Huang, Y.-T.; Chen, Y.-L.; Jeng, U.-S.; Lai, Y.-H.; Sun, Y.-S. Morphology and properties of Nafion membranes prepared by solution casting. Polymer 2009, 50, 1764–1777. [Google Scholar] [CrossRef]
- Fontananova, E.; Cucunato, V.; Curcio, E.; Trotta, F.; Biasizzo, M.; Drioli, E.; Barbieri, G. Influence of the preparation conditions on the properties of polymeric and hybrid cation exchange membranes. Electrochim. Acta 2012, 66, 164–172. [Google Scholar] [CrossRef]
- Kaliaguine, S.; Mikhailenko, S.D.; Wang, K.P.; Xing, P.; Robertson, G.; Guiver, M. Properties of SPEEK based PEMs for fuel cell application. Catal. Today 2003, 82, 213–222. [Google Scholar] [CrossRef]
- Xi, J.; Li, Z.; Yu, L.; Yin, B.; Wang, L.; Liu, L.; Qiu, X.; Chen, L. Effect of degree of sulfonation and casting solvent on sulfonated poly(ether ether ketone) membrane for vanadium redox flow battery. J. Power Sources 2015, 285, 195–204. [Google Scholar] [CrossRef]
- Lu, D.; Babaniamansour, P.; Williams, A.; Opfar, K.; Nurick, P.; Escobar, I.C. Fabrication and evaporation time investigation of water treatment membranes using green solvents and recycled polyethylene terephthalate. J. Appl. Polym. Sci. 2022, 139, e52823. [Google Scholar] [CrossRef]
- Roy, K.-M. Sulfones and Sulfoxides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Portale, G.; Longo, A.; D’Ilario, L.; Passalacqua, E. Sulphonated poly(ether ether ketone) membranes for fuel cell application: Thermal and structural characterisation. J. Power Sources 2006, 163, 18–26. [Google Scholar] [CrossRef]
- Cseri, L.; Szekely, G. Towards cleaner PolarClean: Efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. Green Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef]
- Kong, D.; Dolzhenko, A.V. Cyrene: A bio-based sustainable solvent for organic synthesis. Sustain. Chem. Pharm. 2022, 25, 100591. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Molino, A.; Figoli, A. New frontiers in sustainable membrane preparation: CyreneTM as green bioderived solvent. J. Memb. Sci. 2019, 580, 224–234. [Google Scholar] [CrossRef]
- Bridge, A.T.; Pedretti, B.J.; Brennecke, J.F.; Freeman, B.D. Preparation of defect-free asymmetric gas separation membranes with dihydrolevoglucosenone (CyreneTM) as a greener polar aprotic solvent. J. Memb. Sci. 2022, 644, 120173. [Google Scholar] [CrossRef]
- Wibisono, Y.; Noviani, V.; Ramadhani, A.T.; Devianto, L.A.; Sulianto, A.A. Eco-friendly forward osmosis membrane manufacturing using dihydrolevoglucosenone. Results Eng. 2022, 16, 100712. [Google Scholar] [CrossRef]
- Milescu, R.A.; Zhenova, A.; Vastano, M.; Gammons, R.; Lin, S.; Lau, C.H.; Clark, J.H.; McElreoy, C.R.; Pellis, A. Polymer Chemistry Applications of Cyrene and its Derivative Cygnet 0.0 as Safer Replacements for Polar Aprotic Solvents. ChemSusChem 2021, 14, 3367–3381. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Simone, S.; Figoli, A. DMSO EVOLTM as novel non-toxic solvent for polyethersulfone membrane preparation. Environ. Sci. Pollut. Res. 2019, 26, 14774–14785. [Google Scholar] [CrossRef]
- Evenepoel, N.; Wen, S.; Tilahun Tsehaye, M.; Van der Bruggen, B. Potential of DMSO as greener solvent for PES ultra- and nanofiltration membrane preparation. J. Appl. Polym. Sci. 2018, 135, 46494. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. Use of γ-valerolactone and glycerol derivatives as bio-based renewable solvents for membrane preparation. Green Chem. 2019, 21, 1054–1064. [Google Scholar] [CrossRef]
- Lau, S.K.; Jia, T.-Z.; Cao, X.-L.; Sun, S.-P.; Yong, W.F. Sustainable fabrication of zwitterionic nanofiltration membranes with enhanced antifouling performance using sugar. J. Environ. Chem. Eng. 2023, 11, 110588. [Google Scholar] [CrossRef]
- Saïdi, S.; Macedonio, F.; Russo, F.; Hannachi, C.; Hamrouni, B.; Drioli, E.; Figoli, A. Preparation and characterization of hydrophobic P(VDF-HFP) flat sheet membranes using Tamisolve® NxG solvent for the treatment of saline water by direct contact membrane distillation and membrane crystallization. Sep. Purif. Technol. 2021, 275, 119144. [Google Scholar] [CrossRef]
- Russo, F.; Marino, T.; Galiano, F.; Gzara, L.; Gordano, A.; Organji, H.; Figoli, A. Tamisolve® NxG as an Alternative Non-Toxic Solvent for the Preparation of Porous Poly (Vinylidene Fluoride) Membranes. Polymers 2021, 13, 2579. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, C.; Op de Beeck, D.; Ilyas, A.; Thijs, M.; Koeckelbergsh, G.; Aerts, P.E.M.; Vankelecom, I.F.J. Ultra-thin and highly porous PVDF-filters prepared via phase inversion for potential medical (COVID-19) and industrial use. J. Memb. Sci. 2021, 639, 119710. [Google Scholar] [CrossRef]
- Alqadhi, N.; Abdellah, M.H.; Nematulloev, S.; Mohammed, O.F.; Abdulhamid, M.A.; Szekely, G. Solution-processable poly(ether-ether-ketone) membranes for organic solvent nanofiltration: From dye separation to pharmaceutical purification. Sep. Purif. Technol. 2024, 328, 125072. [Google Scholar] [CrossRef]
- Dedvukaj, A.; Van den Mooter, P.; Vankelecom, I.F.J. Solvent-Resistant UV-Cured Polysulfone Support Membranes Using a Green Solvent. Membranes 2021, 12, 1. [Google Scholar] [CrossRef]
- Bagnato, G.; Figoli, A.; Garbe, R.; Russo, F.; Galiano, F.; Sanna, A. Development of Ru-PEEK-WC catalytic membrane using a more sustainable solvent for stable hydrogenation reactions. Fuel Process. Technol. 2021, 216, 106766. [Google Scholar] [CrossRef]
- Marino, T.; Blasi, E.; Tornaghi, S.; Di Nicolò, E.; Figoli, A. Polyethersulfone membranes prepared with Rhodiasolv®Polarclean as water soluble green solvent. J. Memb. Sci. 2018, 549, 192–204. [Google Scholar] [CrossRef]
- Wang, H.H.; Jung, J.T.; Kim, J.F.; Kim, S.; Drioli, E.; Lee, Y.M. A novel green solvent alternative for polymeric membrane preparation via nonsolvent-induced phase separation (NIPS). J. Memb. Sci. 2019, 574, 44–54. [Google Scholar] [CrossRef]
- Russo, F.; Castro-Muñoz, R.; Galiano, F.; Figoli, A. Unprecedented preparation of porous Matrimid® 5218 membranes. J. Memb. Sci. 2019, 585, 166–174. [Google Scholar] [CrossRef]
- Dong, X.; Shannon, H.D.; Parker, C.; De Jesus, S.; Escobar, I.C. Comparison of two low-hazard organic solvents as individual and cosolvents for the fabrication of polysulfone membranes. AIChE J. 2020, 66, e16790. [Google Scholar] [CrossRef]







| 12 Principles of Green Chemistry of Anastas and Warner |
|---|
| Waste prevention: Better avoid the waste than treat it afterwards. |
| Atom economy: Use the maximum number of reagent atoms in your product to reduce waste. |
| Less hazardous chemical synthesis: Minimize substances’ hazards during reactions and waste. |
| Designing safer chemicals: Minimize the toxicity directly by molecular design. |
| Safer solvents and auxiliaries: Choose as few as possible solvents and auxiliaries and choose the safest solvent. |
| Design for energy efficiency: Choose the least energy-intensive chemical route. |
| Use of renewable feedstocks: Use as much as possible renewable sources and as few as possible petrochemical sources. |
| Reduce derivatives: Minimize the use of temporary derivatives such as protecting groups. |
| Catalysis: Use catalytic reactions to help increase selectivity, minimize waste and reduce reaction times and energy demands. |
| Design for degradation: Design chemicals that can degrade and thus be discarded easily. |
| Real-time pollution prevention: Monitor the processes in real time to prevent the release of hazardous and polluting substances. |
| 10 objectives of green and sustainable chemistry of the United Nations |
| Minimizing Chemical Hazards: Design of chemicals with minimized (or no) hazard properties for use in materials, products and production processes (“benign by design”). |
| Avoiding regrettable substitutions and alternatives: Develop safe and sustainable alternatives for chemicals of concern through material and product innovations that do not create negative trade-offs. |
| Sustainable sourcing of resources and feedstocks: Use of sustainably sourced resources, materials and feedstocks without creating negative trade-offs. |
| Advancing Sustainability of Production Processes: Use green and sustainable chemistry innovation to improve resource efficiency, pollution prevention, and waste minimization in industrial processes. |
| Advancing Sustainability of Products: Use green and sustainable chemistry innovation to create sustainable products and consumption with minimized (or no) chemical hazard potential. |
| Minimize chemical release and pollution: Reduce chemical releases throughout the life cycle of chemicals and products. |
| Enabling non-toxic circularity and minimizing waste: Use of chemistry innovations to enable non-toxic circular material flows and sustainable supply and value chains throughout the life cycle. |
| Maximizing Social Benefits: Consider social factors, high standards of ethics, education and justice in chemistry innovation. |
| Protecting workers, consumers, and vulnerable populations: Safeguard the health of workers, consumers and vulnerable groups in formal and informal sectors. |
| Developing solutions for sustainability challenges: Focus on chemistry innovation to help address societal and sustainability challenges. |
| Solvent | Main Polymer | Brief Description | Refs. |
|---|---|---|---|
| Cyrene™ | PES and PVDF | Porous membranes via phase inversion | [153] |
| PSF | Dense asymmetric gas separation membranes | [154] | |
| Cellulose, CA 1 | Membrane via phase inversion | [155] | |
| Cyrene and Cygnet 0.0 2 | CA, PSF and polyimides | Flat sheet membranes using pure cyrene, pure cygnet or a blend of the two. | [156] |
| DMSO | PES | Ultrafiltration and nanofiltration membranes | [157,158] |
| OPBI 3 | Sulfonation step performed with DMSO and CEM cast from a 12 wt% casting solution | [52] | |
| PEEK-WC | Comparison of DMAc and DMSO and varying multiple membrane synthesis parameters | [145] | |
| Nafion polymer | Influence of solubility parameters and dielectric constant | [144] | |
| sPEEK | Synthesis CEM | [146,150] | |
| GVL | CA, CTA 4, PI, PES and PSF | Membrane preparation via phase inversion | [159] |
| TamiSolve NxG | PES | Synthesis of porous membranes | [138,160] |
| PVDF-HFP 5 | Porous membranes for membrane distillation | [143,161] | |
| PVDF | Porous membranes | [162,163] | |
| PEEK | Membrane for organic solvent nanofiltration | [164] | |
| PSF | Synthesis of a crosslinked support | [165] | |
| PEEK-WC | Synthesis catalytic membranes | [166] | |
| Rhodiasolv® PolarClean | PES | Ultrafiltration and nanofiltration membranes | [167] |
| PES, PSF and CA | Ultrafiltration and nanofiltration membranes | [168] | |
| Matrimid® | Microfiltration and ultrafiltration membranes | [169] | |
| Polarclean and GVL | PSF | Ultrafiltration membranes | [170] |
| PES and PET | Ultrafiltration membranes | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depuydt, S.; Van der Bruggen, B. Green Synthesis of Cation Exchange Membranes: A Review. Membranes 2024, 14, 23. https://doi.org/10.3390/membranes14010023
Depuydt S, Van der Bruggen B. Green Synthesis of Cation Exchange Membranes: A Review. Membranes. 2024; 14(1):23. https://doi.org/10.3390/membranes14010023
Chicago/Turabian StyleDepuydt, Stef, and Bart Van der Bruggen. 2024. "Green Synthesis of Cation Exchange Membranes: A Review" Membranes 14, no. 1: 23. https://doi.org/10.3390/membranes14010023
APA StyleDepuydt, S., & Van der Bruggen, B. (2024). Green Synthesis of Cation Exchange Membranes: A Review. Membranes, 14(1), 23. https://doi.org/10.3390/membranes14010023