ZnO Microfiltration Membranes for Desalination by a Vacuum Flow-Through Evaporation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atomic Layer Deposition Process
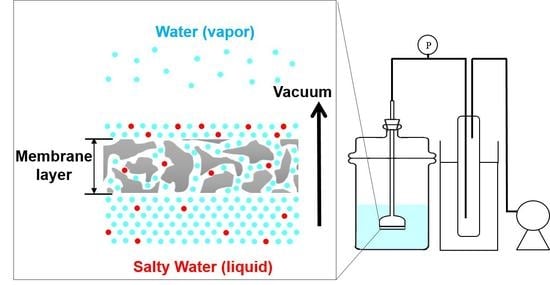
2.2. Membrane Desalination Tests
2.3. Membrane Characterization
3. Results and Discussion
3.1. ZnO ALD Membrane Characterization
3.2. ZnO ALD Membrane Performance
3.3. Effect of Ultrasonic Membrane Cleaning
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Liu, R.; Young, S.; Dangwal, S.; Shaik, I.; Echeverria, E.; McIlroy, D.; Aichele, C.; Kim, S.-J. Boron substituted MFI-type zeolite-coated mesh for oil-water separation. Colloids Surf. A 2018, 550, 108–114. [Google Scholar] [CrossRef]
- Liu, R.; Dangwal, S.; Shaik, I.; Aichele, C.; Kim, S.-J. Hydrophilicity-controlled MFI-type zeolite-coated mesh for oil/water separation. Sep. Purif. Technol. 2018, 195, 163–169. [Google Scholar] [CrossRef]
- Charcosset, C. A review of membrane processes and renewable energies for desalination. Desalination 2009, 245, 214–231. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Huang, A.; Feng, B. Synthesis of novel graphene oxide-polyimide hollow fiber membranes for seawater desalination. J. Membr. Sci. 2018, 548, 59–65. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; Diniz da Costa, J.C.; Wang, D.K. Performance and long term stability of mesoporous silica membranes for desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Coating techniques for membrane distillation: An experimental assessment. Sep. Purif. Technol. 2018, 193, 38–48. [Google Scholar] [CrossRef]
- Garofalo, A.; Donato, L.; Drioli, E.; Criscuoli, A.; Carnevale, M.; Alharbi, O.; Aljlil, S.; Algieri, C. Supported MFI zeolite membranes by cross flow filtration for water treatment. Sep. Purif. Technol. 2014, 137, 28–35. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Faki, B.; Luque-Alled, J.M.; Alberto, M.; Vijayaraghavan, A.; Holmes, S.M.; Szekely, G.; Badawy, M.I.; Shokri, N. Flux-enhanced PVDF mixed matrix membranes incorporating APTS-functionalized graphene oxide for membrane distillation. J. Membr. Sci. 2018, 554, 309–323. [Google Scholar] [CrossRef]
- Bush, J.A.; Vanneste, J.; Gustafson, E.M.; Waechter, C.A.; Jassby, D.; Turchi, C.S.; Cath, T.Y. Prevention and management of silica scaling in membrane distillation using pH adjustment. J. Membr. Sci. 2018, 554, 366–377. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N.R. Polymeric nanocomposite membranes for next generation pervaporation process: Strategies, challenges and future prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; da Costa, J.C.D. Interlayer-free P123 carbonised template silica membranes for desalination with reduced salt concentration polarisation. J. Membr. Sci. 2015, 475, 376–383. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; Motuzas, J.; da Costa, J.C.D. High performance interlayer-free mesoporous cobalt oxide silica membranes for desalination applications. Desalination 2015, 365, 308–315. [Google Scholar] [CrossRef]
- Yacou, C.; Smart, S.; da Costa, J.C.D. Mesoporous TiO2 based membranes for water desalination and brine processing. Sep. Purif. Technol. 2015, 147, 166–171. [Google Scholar] [CrossRef]
- Song, Y.; Wang, D.K.; Birkett, G.; Martens, W.; Duke, M.C.; Smart, S.; da Costa, J.C.D. Mixed matrix carbon molecular sieve and alumina (CMS-Al2O3) membranes. Sci. Rep. 2016, 6, 30703–30710. [Google Scholar] [CrossRef]
- Swenson, P.; Tanchuk, B.; Gupta, A.; An, W.; Kuznicki, S.M. Pervaporative desalination of water using natural zeolite membranes. Desalination 2012, 285, 68–72. [Google Scholar] [CrossRef]
- Makanjuola, O.; Janajreh, I.; Hashaikeh, R. Novel technique for fabrication of electrospun membranes with high hydrophobicity retention. Desalination 2018, 436, 98–106. [Google Scholar] [CrossRef]
- Qiu, H.; Peng, Y.; Ge, L.; Hernandez, B.V.; Zhu, Z. Pore channel surface modification for enhancing anti-fouling membrane distillation. Appl. Surf. Sci. 2018, 443, 217–226. [Google Scholar] [CrossRef]
- Knez, M.; Nielsch, K.; Niinistö, L. Synthesis and surface engineering of complex nanostructures by atomic layer deposition. Adv. Mater. 2007, 19, 3425–3438. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin solid Film. 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Elam, J.; Routkevitch, D.; Mardilovich, P.; George, S. Conformal coating on ultrahigh-aspect-ratio nanopores of anodic alumina by atomic layer deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Kucheyev, S.; Biener, J.; Wang, Y.; Baumann, T.; Wu, K.; Van Buuren, T.; Hamza, A.; Satcher, J., Jr.; Elam, J.; Pellin, M. Atomic layer deposition of ZnO on ultralow-density nanoporous silica aerogel monoliths. Appl. Phys. Lett. 2005, 86, 083108. [Google Scholar] [CrossRef]
- Elam, J.; Libera, J.; Pellin, M.; Zinovev, A.; Greene, J.; Nolen, J. Atomic layer deposition of W on nanoporous carbon aerogels. Appl. Phys. Lett. 2006, 89, 053124. [Google Scholar] [CrossRef]
- Jia, X.; Low, Z.; Chen, H.; Xiong, S.; Wang, Y. Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances. Chin. J. Chem. Eng. 2018, 26, 695–700. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Wang, X.; Wang, Z.; Jin, W.; Huang, J.; Wang, Y. Atomic layer deposition of alumina on porous polytetrafluoroethylene membranes for enhanced hydrophilicity and separation performances. J. Membr. Sci. 2012, 415, 435–443. [Google Scholar] [CrossRef]
- Alam, J.; Alhoshan, M.; Dass, L.A.; Shukla, A.K.; Muthumareeswaran, M.; Hussain, M.; Aldwayyan, A.S. Atomic layer deposition of TiO2 film on a polyethersulfone membrane: Separation applications. J. Polym. Res. 2016, 23, 183. [Google Scholar] [CrossRef]
- Juholin, P.; Kääriäinen, M.-L.; Riihimäki, M.; Sliz, R.; Aguirre, J.L.; Pirilä, M.; Fabritius, T.; Cameron, D.; Keiski, R.L. Comparison of ALD coated nanofiltration membranes to unmodified commercial membranes in mine wastewater treatment. Sep. Purif. Technol. 2018, 192, 69–77. [Google Scholar] [CrossRef]
- Song, Z.; Fathizadeh, M.; Huang, Y.; Chu, K.H.; Yoon, Y.; Wang, L.; Xu, W.L.; Yu, M. TiO2 nanofiltration membranes prepared by molecular layer deposition for water purification. J. Membr. Sci. 2016, 510, 72–78. [Google Scholar] [CrossRef]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond Sci. Tech. 2014, 29, 043001–043009. [Google Scholar] [CrossRef]
- Giovambattista, N.; Debenedetti, P.G.; Rossky, P.J. Effect of surface polarity on water contact angle and interfacial hydration structure. J. Phys. Chem. B 2007, 111, 9581–9587. [Google Scholar] [CrossRef]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, J.; Theato, P.; Yoon, J. Measuring hydrophilicity of RO membranes by contact angles via sessile drop and captive bubble method: A comparative study. Desalination 2012, 303, 23–28. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M. Probing polyamide membrane surface charge, zeta potential, wettability, and hydrophilicity with contact angle measurements. J. Membr. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Hejda, F.; Solar, P.; Kousal, J. Surface free energy determination by contact angle measurements–a comparison of various approaches. WDS 2010, 10, 25–30. [Google Scholar]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process. Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Lin, H.; Liu, R.; Dangwal, S.; Kim, S.-J.; Mehra, N.; Li, Y.; Zhu, J. Permselective H2/CO2 Separation and Desalination of Hybrid GO/rGO Membranes with Controlled Pre-cross-linking. ACS Appl. Mater. Interfaces 2018, 10, 28166–28175. [Google Scholar] [CrossRef]










| Methods | Membrane | Membrane Pore Size (nm) | Salt Concentration (ppm) | Ion Rejection (%) | Flux (L m−2 h−1) | Tfeed (°C) | Tperm (°C) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pervaporation | SiO2 | 1.8 | - | - | 9.5 | 25 | 25 | [7] |
| SiO2 | 1.8–3.1 | - | - | 6.6 | 25 | 25 | [14] | |
| CoO-SiO2 | <10 | - | - | 7.7 | 25 | 25 | [15] | |
| TiO2 | 3.1 | - | - | 6.0 | 25 | 25 | [16] | |
| CMS-Al2O3 | 0.5–7.0 | - | - | 25 | 25 | 25 | [17] | |
| Clinoptilolites zeolite | 0.72 | - | 98.0 | 2.5 | 25 | 25 | [18] | |
| Membrane distillation | Polypropylene membrane | 200 | 21,200 | 99.9 | 11 | 60 | 20 | [11] |
| Electrospun membrane | 1250 | 10,000 | 99.9 | 8.5 | 65 | 25 | [19] | |
| SiO2 composite membrane | 703 | 35,000 | - | 23 | 70 | 25 | [20] | |
| PVDF Mixed matrix membrane | 970 | 35,000 | 99.9 | 6.2 | 80 | 25 | [10] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dangwal, S.; Liu, R.; Bastatas, L.D.; Echeverria, E.; Huang, C.; Mao, Y.; Mcllroy, D.N.; Han, S.; Kim, S.-J. ZnO Microfiltration Membranes for Desalination by a Vacuum Flow-Through Evaporation Method. Membranes 2019, 9, 156. https://doi.org/10.3390/membranes9120156
Dangwal S, Liu R, Bastatas LD, Echeverria E, Huang C, Mao Y, Mcllroy DN, Han S, Kim S-J. ZnO Microfiltration Membranes for Desalination by a Vacuum Flow-Through Evaporation Method. Membranes. 2019; 9(12):156. https://doi.org/10.3390/membranes9120156
Chicago/Turabian StyleDangwal, Shailesh, Ruochen Liu, Lyndon D. Bastatas, Elena Echeverria, Chengqian Huang, Yu Mao, David N. Mcllroy, Sangil Han, and Seok-Jhin Kim. 2019. "ZnO Microfiltration Membranes for Desalination by a Vacuum Flow-Through Evaporation Method" Membranes 9, no. 12: 156. https://doi.org/10.3390/membranes9120156
APA StyleDangwal, S., Liu, R., Bastatas, L. D., Echeverria, E., Huang, C., Mao, Y., Mcllroy, D. N., Han, S., & Kim, S. -J. (2019). ZnO Microfiltration Membranes for Desalination by a Vacuum Flow-Through Evaporation Method. Membranes, 9(12), 156. https://doi.org/10.3390/membranes9120156