A Bibliometric Survey of Paraffin/Olefin Separation Using Membranes
Abstract
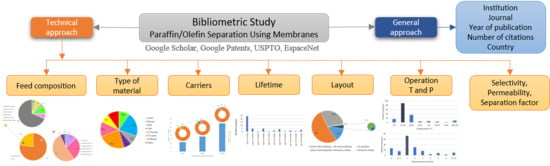
:1. Introduction
2. Data Sources and Methodology
3. Results
3.1. The Annual Distribution
3.2. The Scientific Journals Distribution
3.3. The Country and Institutions Distribution
3.4. The Most Cited Papers
3.5. The Separated Streams
3.6. The Used Membranes
3.7. The Carrier Agents
3.8. The Poisonous Agents and the Lifetime
3.9. The Layouts and Operation Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eldridge, R.B. Olefin/paraffin separation technology: a review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Rungta, M.; Zhang, C.; Koros, W.J.; Xu, L. Membrane-based ethylene/ethane separation: The upper bound and beyond. AIChE J. 2013, 59, 3475–3489. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3. [Google Scholar] [CrossRef]
- Ferraz, H.C.; Duarte, L.T.; Di Luccio, M.; Alves, T.L.M.; Habert, A.C.; Borges, C.P. Recent achievements in facilitated transport membranes for separation processes. Braz. J. Chem. Eng. 2007, 4, 101–118. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Godini, H.R.; Kim, M.; Görke, O.; Khadivi, M.; Schomäcker, R.; Repke, J.U. Membrane Engineering for the Treatment of Gases: Gas-separation Issues Combined with Membrane Reactors; Royal Society of Chemistry: London, UK, 2011; ISBN 978-1849732390. [Google Scholar]
- Hou, J.; Liu, P.; Jiang, M.; Yu, L.; Li, L.; Tang, Z. Olefin/paraffin separation through membranes: from mechanisms to critical materials. J. Mater. Chem. A 2019, 7, 23489–23511. [Google Scholar] [CrossRef]
- Norwahyu, J.; Lau, K.K.; Yeong, Y.F.; Shariff, A.M. Bulk CO2/CH4 Separation for Offshore Operating Conditions using Membrane Process. Sains Malaysiana 2016, 45, 1707–1714. [Google Scholar]
- Ismail, N.M.; Ismail, A.F.; Mustaffa, A. Characterization of polyethersulfone/cloisite 15A mixed matrix membrane for CO2/CH4 separation. J. Teknol. (Sci. Eng.) 2014, 69, 83–87. [Google Scholar] [CrossRef]
- Doğu, M.; Ercan, N. High performance cyclic olefin copolymer (COC) membranes prepared with melt processing method and using of surface modified graphitic nano-sheets for H2/CH4 and H2/CO2 separation. Chem. Eng. Res. Des. 2016, 109, 455–463. [Google Scholar] [CrossRef]
- Wiheeb, A.D.; Kim, J.; Othman, M.R. Highly Perm-Selective Micro-Porous Hydrotalcite-Silica Membrane for Improved Carbon Dioxide-Methane Separation. Sep. Sci. Technol. 2015, 50, 1701–1708. [Google Scholar] [CrossRef]
- Han, Y.J.; Ko, K.J.; Choi, H.K.; Moon, J.H.; Lee, C.H. Kinetic effects of methane on binary mixture separation on methyltriethoxysilane templated silica membranes. Sep. Purif. Technol. 2017, 182, 151–159. [Google Scholar] [CrossRef]
- Khoshkharam, A.; Ghayyem, M.A.; Behbahani, R.M. Laboratory investigation of carbon dioxide separation from methane using a PES/Pebax 1657 composite membrane. Pet. Sci. Technol. 2017, 35, 471–478. [Google Scholar] [CrossRef]
- Shamsabadi, A.A.; Kargari, A.; Farshadpour, F.; Laki, S. Mathematical Modeling of CO2/CH4 Separation by Hollow Fiber Membrane Module Using Finite Difference Method. J. Membr. Sep. Technol. 2012, 1, 19–29. [Google Scholar]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Microwave heating-synthesized zeolite membrane for CO2/CH4 separation. Desalin. Water Treat. 2012, 47, 139–149. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Hamrahi, Z.; Kargari, A. Modification of polycarbonate membrane by polyethylene glycol for CO2/CH4 separation. Sep. Sci. Technol. 2017, 52, 544–556. [Google Scholar] [CrossRef]
- Askari, M.; Yang, T.; Chung, T.S. Natural gas purification and olefin/paraffin separation using cross-linkable dual-layer hollow fiber membranes comprising Β-Cyclodextrin. J. Membr. Sci. 2012, 423, 392–403. [Google Scholar] [CrossRef]
- Isanejad, M.; Azizi, N.; Mohammadi, T. Pebax membrane for CO2/CH4 separation: Effects of various solvents on morphology and performance. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, F.; Thomas, S.; Zhu, G.; Valtchev, V.; Mintova, S. Co3(HCOO)6 Microporous Metal-Organic Framework Membrane for Separation of CO2/CH4 Mixtures. Chem. - A Eur. J. 2011, 17, 12076–12083. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Performance and plasticization behavior of polymer-MOF membranes for gas separation at elevated pressures. J. Membr. Sci. 2014, 470, 166–177. [Google Scholar] [CrossRef]
- Saedi, S.; Seidi, F.; Moradi, F.; Xiang, X. Preparation and characterization of an amino-cellulose (AC) derivative for development of thin-film composite membrane for CO2/CH4 separation. Starch/Staerke 2016, 68, 651–661. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, N.; Wang, H.; Bu, N.; Zhang, F.; Zhou, R. Preparation of steam-stable high-silica CHA (SSZ-13) membranes for CO2/CH4 and C2H4/C2H6 separation. J. Membr. Sci. 2015, 475, 303–310. [Google Scholar] [CrossRef]
- Han, Y.J.; Kang, J.H.; Kim, H.E.; Moon, J.H.; Cho, C.H.; Lee, C.H. Separation of Carbon Dioxide and Methane Mixture by an Adsorbent/Membrane Hybrid System Using Zeolite 5A Pellets and FAU-Zeolite Membrane. Ind. Eng. Chem. Res. 2017, 56, 2582–2591. [Google Scholar] [CrossRef]
- Atchariyawut, S.; Jiraratananon, R.; Wang, R. Separation of CO2 from CH4 by using gas-liquid membrane contacting process. J. Membr. Sci. 2007, 304, 163–172. [Google Scholar] [CrossRef]
- Ismail, N.; Salleh, W.; Sazali, N.; Ismail, A. The Effect of Polymer Composition on CO2/CH4 Separation of Supported Carbon Membrane. Chem. Eng. Trans. 2015, 45, 1465–1470. [Google Scholar]
- Lai, L.S.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Single and Binary CO2/CH4 Separation of a Zeolitic Imidazolate Framework-8 Membrane. Chem. Eng. Technol. 2017, 40, 1031–1042. [Google Scholar] [CrossRef]
- Chultheera, P.; Rirksomboon, T.; Kulprathipanja, S.; Liu, C.; Chinsirikul, W.; Kerddonfag, N. Solid-Liquid-Polymer Mixed Matrix Membrane Using Liquid Additive Adsorbed on Activated Carbon Dispersed in Polymeric Membrane for CO2/CH4 Separation. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2017, 11, 425–428. [Google Scholar]
- Himeno, S.; Tomita, T.; Suzuki, K.; Nakayama, K.; Yajima, K.; Yoshida, S. Synthesis and Permeation Properties of a DDR-Type Zeolite Membrane for Separation of CO2/CH4 Gaseous Mixtures. Ind. Eng. Chem. Res. 2007, 46, 6989–6997. [Google Scholar] [CrossRef]
- Khoshkam, M.; Sadeghi, M.; Chenar, M.P.; Namazifard, M.J. Synthesis of polyimide membrane for the separation of CO2/CH4 gases Chemical Engineering Department. In Proceedings of the Polymer Processing Society Asia/Australia Regional Meeting, Mashhad, Iran, 15–17 November 2011; pp. 15–18. [Google Scholar]
- Adewole, J.K.; Ahmad, A.L.; Ismail, S.; Leo, C.P.; Sultan, A.S. Comparative studies on the effects of casting solvent on physico-chemical and gas transport properties of dense polysulfone membrane used for CO2/CH4 separation. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Kim, S.; Ko, D.; Row, S.; Kim, J. Techno-economic evaluation of hybrid systems of pressure swing adsorption and membrane processes for coalbed methane separation. Chem. Eng. Res. Des. 2016, 115, 230–240. [Google Scholar] [CrossRef]
- Saedi, S.; Madaeni, S.S.; Hassanzadeh, K.; Shamsabadi, A.A.; Laki, S. The effect of polyurethane on the structure and performance of PES membrane for separation of carbon dioxide from methane. J. Ind. Eng. Chem. 2014, 20, 1916–1929. [Google Scholar] [CrossRef]
- Saedi, S.; Madaeni, S.S.; Arabi Shamsabadi, A.; Mottaghi, F. The effect of surfactants on the structure and performance of PES membrane for separation of carbon dioxide from methane. Sep. Purif. Technol. 2012, 99, 104–119. [Google Scholar] [CrossRef]
- Zahri, K.; Goh, P.S.; Ismail, A.F. The incorporation of graphene oxide into polysulfone mixed matrix membrane for CO2/CH4 separation. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012007. [Google Scholar] [CrossRef]
- Van Kemenade, H.P.; van Benthum, R.J.; Brouwers, J.J.H. Upgrading Carbon Dioxide/Methane Mixtures by using a Hybrid Membrane-Condensed Rotational Separation Process. Energy Technol. 2014, 2, 874–876. [Google Scholar] [CrossRef]
- Lai, L.S.; Yeong, Y.F.; Keong Lau, K.; Azmi, M.S. Zeolitic Imidazolate Frameworks (ZIF): A Potential Membrane for CO2/CH4 Separation. Sep. Sci. Technol. 2014, 49, 1490–1508. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chai, S.P.; Zhu, P.W.; Mohamed, A.R. Development of a hybrid membrane through coupling of high selectivity zeolite T on ZIF-8 intermediate layer and its performance in carbon dioxide and methane gas separation. Microporous Mesoporous Mater. 2014, 196, 79–88. [Google Scholar] [CrossRef]
- Chen, D.L.; Shang, H.; Zhu, W.; Krishna, R. Reprint of: Transient breakthroughs of CO2/CH4 and C3H6/C3H8 mixtures in fixed beds packed with Ni-MOF-74. Chem. Eng. Sci. 2015, 124, 109–117. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Mukhtar, H.; Man, Z. Composite blending of ionic liquid–poly(ether sulfone) polymeric membranes: Green materials with potential for carbon dioxide/methane separation. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Development and performance evaluation of Polydimethyl siloxane/Polysulfone (PDMS/PSF) composite membrane for CO2/CH4 separation. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012014. [Google Scholar] [CrossRef] [Green Version]
- Gamali, P.A.; Kazemi, A.; Zadmard, R.; Anjareghi, M.J.; Rezakhani, A.; Rahighi, R.; Madani, M. Distinguished discriminatory separation of CO2 from its methane-containing gas mixture via PEBAX mixed matrix membrane. Chinese J. Chem. Eng. 2018, 26, 137–143. [Google Scholar] [CrossRef]
- Hasan, R.; Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Effect of Hydrocarbons on the separation of carbon dioxide from methane through a polyimide gas separation membrane. Ind. Eng. Chem. Res. 2009, 48, 5415–5419. [Google Scholar] [CrossRef]
- Deng, L.; Hägg, M.-B. Fabrication and Evaluation of a Blend Facilitated Transport Membrane for CO2/CH4 Separation. Ind. Eng. Chem. Res. 2015, 54, 11139–11150. [Google Scholar] [CrossRef]
- Mohamad, M.B.; Fong, Y.Y.; Shariff, A. Gas Separation of Carbon Dioxide from Methane Using Polysulfone Membrane Incorporated with Zeolite-T. Procedia Eng. 2016, 148, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ding, X.; Yang, P.; Li, L.; Li, X.; Zhang, Y. A novel multi-armed and star-like poly(ethylene oxide) membrane for CO2 separation. J. Membr. Sci. 2015, 489, 258–263. [Google Scholar] [CrossRef]
- Erdni-Goryaev, E.M.; Alent’ev, A.Y.; Belov, N.A.; Ponkratov, D.O.; Shaplov, A.S.; Lozinskaya, E.I.; Vygodskii, Y.S. Gas separation characteristics of new membrane materials based on poly(ethylene glycol)-crosslinked polymers and ionic liquids. Pet. Chem. 2012, 52, 494–498. [Google Scholar] [CrossRef]
- Rouleau, L.; Pirngruber, G.; Guillou, F.; Barrère-Tricca, C.; Omegna, A.; Valtchev, V.; Pera-Titus, M.; Miachon, S.; Dalmon, J.A. Nanocomposite MFI-alumina and FAU-alumina Membranes: Synthesis, Characterization and Application to Paraffin Separation and CO2 Capture. Oil Gas Sci. Technol. - Rev. l’IFP 2009, 64, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Hua, M.M.; Zhao, H.; Yang, P.; Chen, X.; Xin, Q.; Zhang, Y. Poly (ethylene oxide) composite membrane synthesized by UV-initiated free radical photopolymerization for CO2 separation. J. Membr. Sci. 2017, 531, 129–137. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Hua, K.; Deng, M. Poly(amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem. 2014, 23, 227–234. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Zhang, Y.; Bai, Y.; Gu, J.; Sun, Y. Poly(ether-b-amide)/ethylene glycol monophenyl ether gel membrane with superior CO2/N2separation performance fabricated by thermally induced phase separation method. J. Membr. Sci. 2016, 508, 136–145. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: a review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of Membrane Science Development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Campos, A.C.C.; Dos Reis, R.A.; Ortiz, A.; Gorri, D.; Ortiz, I. A Perspective of Solutions for Membrane Instabilities in Olefin/Paraffin Separations: A Review. Ind. Eng. Chem. Res. 2018, 57, 10071–10085. [Google Scholar] [CrossRef] [Green Version]
- Hsiue, G.-H.; Yang, J.-S. Novel methods in separation of olefin/paraffin mixtures by functional polymeric membranes. J. Membr. Sci. 1993, 82, 117–128. [Google Scholar] [CrossRef]
- Ma, X.; Liu, D. Zeolitic imidazolate framework membranes for light olefin/paraffin separation. Crystals 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Rungta, M.; Wenz, G.B.; Zhang, C.; Xu, L.; Qiu, W.; Adams, J.S.; Koros, W.J. Carbon molecular sieve structure development and membrane performance relationships. Carbon 2017, 115, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Lin, Y.S.; Wei, X.; Kniep, J. Ultrathin carbon molecular sieve membrane for propylene/propane separation. AIChE J. 2016, 62, 491–499. [Google Scholar] [CrossRef]
- Tanco, M.A.L.; Tanaka, D.A.P. Recent advances on carbon molecular sieve membranes (CMSMs) and reactors. Processes 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Faiz, R.; Li, K. Polymeric membranes for light olefin/paraffin separation. Desalination 2012, 287, 82–97. [Google Scholar] [CrossRef]
- Al-Maythalony, B.A. Metal-organic framework based membranes for gas separation. In Advanced Nanomaterials for Membrane Synthesis and its Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–226. ISBN 9780128145036. [Google Scholar]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Peh, S.B.; Zhao, D. Alternatives to Cryogenic Distillation: Advanced Porous Materials in Adsorptive Light Olefin/Paraffin Separations. Small 2019. [Google Scholar] [CrossRef] [PubMed]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705. [Google Scholar] [CrossRef]
- Pabby, A.K.; Rizvi, S.S.H.; Sastre, A.M. Handbook of membrane separations: Chemical, pharmaceutical, food, and biotechnological applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466555587. [Google Scholar]
- Stern, E.W. Olefin-Paraffin Separation by Supported Cuprous Chloride. Ind. Eng. Chem. Process Des. Dev. 1962, 1, 281–284. [Google Scholar] [CrossRef]
- Ho, W.S.W.; Doyle, G.; Savage, D.W.; Pruett, R.L. Olefin separations via complexation with cuprous diketonate. Ind. Eng. Chem. Res. 1988, 27, 334–337. [Google Scholar] [CrossRef]
- Le, M.T. An assessment of the potential for the development of the shale gas industry in countries outside of North America. Heliyon 2018, 4, e00516. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Himsl, D.; Kunz, S.; Tangermann, O. Olefin/paraffin separation over the Metal Organic Framework material Cu3(BTC)2; Elsevier B.V.: Amsterdam, The Netherlands, 2008; Volume 174, ISBN 9780444532961. [Google Scholar]
- Lamia, N.; Jorge, M.; Granato, M.A.; Almeida Paz, F.A.; Chevreau, H.; Rodrigues, A.E. Adsorption of propane, propylene and isobutane on a metal-organic framework: Molecular simulation and experiment. Chem. Eng. Sci. 2009, 64, 3246–3259. [Google Scholar] [CrossRef] [Green Version]
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/Ethene Separation Turned on Its Head: Selective Ethane Adsorption on the Metal−Organic Framework ZIF-7 through a Gate-Opening Mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef] [PubMed]
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethene/ethane separation by the MOF membrane ZIF-8: Molecular correlation of permeation, adsorption, diffusion. J. Membr. Sci. 2011, 369, 284–289. [Google Scholar] [CrossRef]
- Ferreira, A.F.P.; Santos, J.C.; Plaza, M.G.; Lamia, N.; Loureiro, J.M.; Rodrigues, A.E. Suitability of Cu-BTC extrudates for propane-propylene separation by adsorption processes. Chem. Eng. J. 2011, 167, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Liu, G.; Belmabkhout, Y.; Adil, K.; Eddaoudi, M.; Koros, W. Conformation-Controlled Molecular Sieving Effects for Membrane-Based Propylene/Propane Separation. Adv. Mater. 2019. [Google Scholar] [CrossRef]
- Hayashi, J.; Mizuta, H.; Yamamoto, M.; Kusakabe, K.; Morooka, S.; Suh, S.-H. Separation of Ethane/Ethylene and Propane/Propylene Systems with a Carbonized BPDA-pp ′ ODA Polyimide Membrane. Ind. Eng. Chem. Res. 1996, 35, 4176–4181. [Google Scholar] [CrossRef]
- Okamoto, K.; Kawamura, S.; Yoshino, M.; Kita, H.; Hirayama, Y.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation through carbonized membranes derived from an asymmetric polyimide hollow fiber membrane. Ind. Eng. Chem. Res. 1999, 38, 4424–4432. [Google Scholar] [CrossRef]
- Menendez, I.; Fuertes, A.B. Aging of carbon membranes under different environments. Carbon 2001, 39, 733–740. [Google Scholar] [CrossRef]
- Yoshino, M.; Nakamura, S.; Kita, H.; Okamoto, K.I.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation performance of carbonized membranes derived from an asymmetric hollow fiber membrane of 6FDA/BPDA-DDBT copolyimide. J. Membr. Sci. 2003, 215, 169–183. [Google Scholar] [CrossRef]
- Curbelo, S.; Müller, E.A. Modelling of Ethane/Ethylene Separation Using Microporous Carbon. Adsorpt. Sci. Technol. 2005, 23, 855–866. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhou, W.; Honda, T.; Tanaka, K.; Kita, H.; Okamoto, K.I. Preparation and gas separation performance of flexible pyrolytic membranes by low-temperature pyrolysis of sulfonated polyimides. J. Membr. Sci. 2005, 261, 17–26. [Google Scholar] [CrossRef]
- Karunaweera, C.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Fabrication and characterization of aging resistant carbon molecular sieve membranes for C3 separation using high molecular weight crosslinkable polyimide, 6FDA-DABA. J. Membr. Sci. 2019, 581, 430–438. [Google Scholar] [CrossRef]
- Graham, T. On the absorption and dialytic separation of gases by colloid septa. J. Franklin Inst. 1867, 83, 39–41. [Google Scholar] [CrossRef]
- Baker, R.W. Gas Separation. In Membrane Technology and Applications; Wiley: Newark, CA, USA, 2012; pp. 325–378. ISBN 978-0-470-74372-0. [Google Scholar]
- Teramoto, M.; Matsuyama, H.; Yamashiro, T.; Okamoto, S. Separation of ethylene from ethane by a flowing liquid membrane using silver nitrate as a carrier. J. Membr. Sci. 1989, 45, 115–136. [Google Scholar] [CrossRef]
- Zastrow, M. Why South Korea is the world’s biggest investor in research. Nature 2016, 534. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Lee, P.S.; Chang, J.-S.; Nam, S.-E.; Park, Y.-I. Preparation of carbon molecular sieve membranes on low-cost alumina hollow fibers for use in C3H6/C3H8 separation. Sep. Purif. Technol. 2018, 194, 443–450. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.W.; Mun, S.H.; Kang, Y.S. Facile synthesis of copper nanoparticles by ionic liquids and its application to facilitated olefin transport membranes. Ind. Eng. Chem. Res. 2009, 48, 7437–7441. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, J.H.; Oh, K.S.; Won, J.; Char, K.; Kim, H.S.; Kang, Y.S. Highly stabilized silver polymer electrolytes and their application to facilitated olefin transport membranes. J. Membr. Sci. 2004, 236, 163–169. [Google Scholar] [CrossRef]
- Kang, S.W.; Lee, D.H.; Park, J.H.; Char, K.; Kim, J.H.; Won, J.; Kang, Y.S. Effect of the polarity of silver nanoparticles induced by ionic liquids on facilitated transport for the separation of propylene/propane mixtures. J. Membr. Sci. 2008, 322, 281–285. [Google Scholar] [CrossRef]
- Kang, S.W.; Hong, J.; Park, J.H.; Mun, S.H.; Kim, J.H.; Cho, J.; Char, K.; Kang, Y.S. Nanocomposite membranes containing positively polarized gold nanoparticles for facilitated olefin transport. J. Membr. Sci. 2008, 321, 90–93. [Google Scholar] [CrossRef]
- Kim, H.S.; Bae, J.Y.; Park, S.J.; Lee, H.; Bae, H.W.; Kang, S.O.; Lee, S.D.; Choi, D.K. Separation of olefin/paraffin mixtures using zwitterionic silver complexes as transport carriers. Chem. - A Eur. J. 2007, 13, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Chun, S.; Kang, Y.S.; Kang, S.W. Durable poly(vinyl alcohol)/AgBF4/Al(NO3)3 complex membrane with high permeance for propylene/propane separation. Sep. Purif. Technol. 2017, 174, 39–43. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.W.; Song, D.; Won, J.; Kang, Y.S. Facilitated olefin transport through room temperature ionic liquids for separation of olefin/paraffin mixtures. J. Membr. Sci. 2012, 423–424, 159–164. [Google Scholar] [CrossRef]
- Jeong, S.; Kang, S.W. Effect of Ag2O nanoparticles on long-term stable polymer/AgBF4/Al(NO3)3 complex membranes for olefin/paraffin separation. Chem. Eng. J. 2017, 327, 500–504. [Google Scholar] [CrossRef]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 2012. [Google Scholar] [CrossRef] [Green Version]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2001, 181, 141. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Li, K.; Olson, D.H.; Seidel, J.; Emge, T.J.; Gong, H.; Zeng, H.; Li, J. Zeolitic imidazolate frameworks for kinetic separation of propane and propene. J. Am. Chem. Soc. 2009, 131, 10368–10369. [Google Scholar] [CrossRef]
- Safarik, D.J.; Eldridge, R.B. Olefin/paraffin separations by reactive absorption: a review. Ind. Eng. Chem. Res. 1998, 37, 2571–2581. [Google Scholar] [CrossRef]
- Liu, C.; Osman, Z. High Hydrocarbon Resistant Chemically Cross-Linked Aromatic Polyimide Membrane For Separations. U.S. Patent 9,296,866 B2, 29 March 2016. [Google Scholar]
- Feng, X.; Huang, R.Y.M. Liquid Separation by Membrane Pervaporation: A Review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Comprehensive Membrane Science and Engineering; Elsevier: Oxford, UK, 2010; ISBN 9780080932507. [Google Scholar]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Merkel, T.; Blanc, R.; Zeid, J.; Suwarlim, A.; Firat, B.; Wijmans, H.; Asaro, M.; Greene, M. Separation of Olefin/Paraffin Mixtures with Carrier Facilitated Membranes; Membrane Technology and Research, Inc.: Menlo Park, CA, USA, 2007. [Google Scholar]
- Jiang, B.; Dou, H.; Wang, B.; Sun, Y.; Huang, Z.; Bi, H.; Zhang, L.; Yang, H. Silver-Based Deep Eutectic Solvents as Separation Media: Supported Liquid Membranes for Facilitated Olefin Transport. ACS Sustain. Chem. Eng. 2017, 5, 6873–6882. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Baertsch, C.; Koval, C.A.; Noble, R.D.; Bowman, C.N. Olefin separation using silver impregnated ion-exchange membranes and silver salt/polymer blend membranes. J. Membr. Sci. 1996, 117, 151–161. [Google Scholar] [CrossRef]
- Kostyanaya, M.; Bazhenov, S.; Borisov, I.; Plisko, T.; Vasilevsky, V. Surface modified polysulfone hollow fiber membranes for ethane/ethylene separation using gas-liquid membrane contactors with ionic liquid-based absorbent. Fibers 2019, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Scholander, P.F. Oxygen Transport through Hemoglobin Solutions. Science 1960, 131, 585–590. [Google Scholar] [CrossRef]
- Merkel, T.C.; Blanc, R.; Ciobanu, I.; Firat, B.; Suwarlim, A.; Zeid, J. Silver salt facilitated transport membranes for olefin/paraffin separations: Carrier instability and a novel regeneration method. J. Membr. Sci. 2013, 447, 177–189. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Rezende, C.G.F. Sorção de Propano e Propeno em Membrana de Poliuretano Contendo Nanopartículas de Prata. Doctorate Dissertation, UFRJ/COPPE, Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Effect of Complexation Reaction Constant on the Separation of Propylene/Propane by Supported Liquid Membrane. J. Membr. Sci. Res. 2015, 1, 85–89. [Google Scholar]
- Schultz, J.S.; Goddard, J.D.; Suchdeo, S.R. Facilitated transport via carrier-mediated diffusion in membranes: Part I. Mechanistic aspects, experimental systems and characteristic regimes. AIChE J. 1974, 20, 417–445. [Google Scholar] [CrossRef]
- Goering, R.M.; Bowman, C.N.; Koval, C.A.; Noble, R.D.; Ashley, M.E. Complexation structure and transport mechanism of 1, 5-hexadiene and 1-hexene through silver facilitated transport membranes. J. Membr. Sci. 2000, 172, 49–57. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Boributh, S.; Jiraratananon, R.; Ortiz, I.; Li, K. Long term stability of PTFE and PVDF membrane contactors in the application of propylene/propane separation using AgNO3 solution. Chem. Eng. Sci. 2013, 94, 108–119. [Google Scholar] [CrossRef]
- King, C.J. Separation Processes Based on Reversible Chemical Complexation. Handbook of Separation Process Technology; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Faiz, R.; Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012, 73, 261–284. [Google Scholar] [CrossRef]
- Jose, B.; Ryu, J.H.; Lee, B.G.; Lee, H.; Kang, Y.S.; Kim, H.S. Effect of phthalates on the stability and performance of AgBF4-PVP membranes for olefin/paraffin separation. Chem. Commun. (Camb). 2001, 20, 2046–2047. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabiah, A. The Use of Hybrid Membrane/Distillation System for the Ethane/Ethylene Separation in Olefin Plants. In Proceedings of the 4th Ibero-American Congress on Membrane Science and Technology (CITEM); 2003; pp. 1–6. [Google Scholar]
- Kuraoka, K.; Matsuura, S.; Ueda, K. Preparation and properties of organic-inorganic hybrid facilitated olefin separation membranes via sol-gel method. Procedia Eng. 2012, 44, 915–917. [Google Scholar] [CrossRef] [Green Version]
- Hamza, A.A.; Towe, I.G. Stable Facilitated Transport Membrane for Olefin/Paraffin Separation. In Proceedings of the AIChE Spring Meeting and Global Congress on Process Safety, San Antonio, TX, USA, 2 May 2013. [Google Scholar]
- Maghsoudi, H. Comparative study of adsorbents performance in ethylene/ethane separation. Adsorption 2016, 22, 985–992. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M.; Ismail, Z. Gas-liquid membrane contactor for ethylene/ethane separation by aqueous silver nitrate solution. Sep. Purif. Technol. 2014, 127, 140–148. [Google Scholar] [CrossRef]
- Nymeijer, K.; Visser, T.; Assen, R.; Wessling, M. Olefin-Selective Membranes in Gas−Liquid Membrane Contactors for Olefin/Paraffin Separation. Ind. Eng. Chem. Res. 2004, 43, 720–727. [Google Scholar] [CrossRef]
- Nymeijer, K.; Visser, T.; Assen, R.; Wessling, M. Super selective membranes in gas-liquid membrane contactors for olefin/paraffin separation. J. Membr. Sci. 2004, 232, 107–114. [Google Scholar] [CrossRef]
- GalánSánchez, L.M.; Meindersma, G.W.; Haan, A. Potential of Silver-Based Room-Temperature Ionic Liquids for Ethylene/Ethane Separation. Ind. Eng. Chem. Res. 2009, 48, 10650–10656. [Google Scholar]
- Sun, Y.; Bi, H.; Dou, H.; Yang, H.; Huang, Z.; Wang, B.; Deng, R.; Zhang, L. A Novel Copper(I)-Based Supported Ionic Liquid Membrane with High Permeability for Ethylene/Ethane Separation. Ind. Eng. Chem. Res. 2017, 56, 741–749. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Polymeric ionic liquid membranes containing IL–Ag+ for ethylene/ethane separation via olefin-facilitated transport. J. Mater. Chem. A 2014, 2, 5631. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, H.; Zhang, L.; Wang, B.; Sun, Y.; Yang, H.; Huang, Z.; Bi, H. Novel supported liquid membranes based on deep eutectic solvents for olefin-paraffin separation via facilitated transport. J. Membr. Sci. 2017, 536, 123–132. [Google Scholar] [CrossRef]
- Nymeijer, D.C.; Visser, T.; Assen, R.; Wessling, M. Composite hollow fiber gas-liquid membrane contactors for olefin/paraffin separation. Sep. Purif. Technol. 2004, 37, 209–220. [Google Scholar] [CrossRef]
- Eriksen, O.I.; Aksnes, E.; Dahl, I.M. Facilitated transport of ethene through Nafion membranes. Part II. Glycerine treated, water swollen membranes. J. Membr. Sci. 1993, 85, 99–106. [Google Scholar] [CrossRef]
- Su, C.; Kuraoka, K.; Yazawa, T. Ethene/Ethane (C2H4/C2H6) Separation through an Inorganic-Organic Hybrid Membrane Containing Silver (I) Ions as Olefin Carriers, Using Poly(N-vinylpyrrolidone) as a Mediation Agent. J. Am. Ceram. Soc. 2001, 84, 654–656. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Solid polymer electrolyte composite membranes for olefin/paraffin separation. J. Membr. Sci. 2001, 184, 39–48. [Google Scholar] [CrossRef]
- Müller, J.; Peinemann, K.V.; Müller, J. Development of facilitated transport membranes for the separation of olefins from gas streams. Desalination 2002, 145, 339–345. [Google Scholar] [CrossRef]
- Teramoto, M.; Takeuchi, N.; Maki, T.; Matsuyama, H. Ethylene/ethane separation by facilitated transport membrane accompanied by permeation of aqueous silver nitrate solution. Sep. Purif. Technol. 2002, 28, 117–124. [Google Scholar] [CrossRef]
- Morisato, A.; He, Z.; Pinnau, I.; Merkel, T.C. Transport properties of PA12-PTMO/AgBF4 solid polymer electrolyte membranes for olefin/paraffin separation. Desalination 2002, 145, 347–351. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Kim, H.S.; Won, J.; Kang, Y.S. Facilitated transport of ethylene across polymer membranes containing silver salt: effect of HBF4 on the photoreduction of silver ions. J. Membr. Sci. 2003, 212, 283–288. [Google Scholar] [CrossRef]
- Teramoto, M.; Shimizu, S.; Matsuyama, H.; Matsumiya, N. Ethylene/ethane separation and concentration by hollow fiber facilitated transport membrane module with permeation of silver nitrate solution. Sep. Purif. Technol. 2005, 44, 19–29. [Google Scholar] [CrossRef]
- Hamouda, S.B.; Nguyen, Q.T.; Langevin, D.; Schaetzel, P.; Roudesli, S. Fine characterization of the ethylene and ethane sorption in poly(amide 12-block-tetramethylenoxide) copolymer/AgBF4 membranes. Eur. Polym. J. 2006, 42, 2994–3005. [Google Scholar] [CrossRef]
- Lee, J.S.; Ko, N.H.; Bae, H.W.; Nguyen, D.Q.; Lee, H.; Choi, D.K.; Cheong, M.; Kim, H.S. Effect of ester group on the performance of zwitterionic imidazolium compounds as membrane materials for separating alkene/alkane mixtures. J. Membr. Sci. 2008, 313, 344–352. [Google Scholar] [CrossRef]
- Kuraoka, K.; Matsuura, S.; Ueda, K. Ethylene/Ethane Separation through a SiO2–Poly(sodium acrylate)–Ag+ Organic–Inorganic Hybrid Membrane. Chem. Lett. 2014, 43, 582–583. [Google Scholar] [CrossRef]
- Ovcharova, A.; Vasilevsky, V.; Borisov, I.; Bazhenov, S.; Volkov, A.; Bildyukevich, A.; Volkov, V. Polysulfone porous hollow fiber membranes for ethylene-ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2017, 183, 162–172. [Google Scholar] [CrossRef]
- Malakhov, A.O.; Bazhenov, S.D.; Vasilevsky, V.P.; Borisov, I.L.; Ovcharova, A.A.; Bildyukevich, A.V.; Volkov, V.V.; Giorno, L.; Volkov, A.V. Thin-film composite hollow fiber membranes for ethylene/ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2019, 219, 64–73. [Google Scholar] [CrossRef]
- Kim, H.S.; Ryu, J.H.; Kim, H.; Ahn, B.S.; Kang, Y.S. Reversible olefin complexation by silver ions in dry poly(vinyl methyl ketone) membrane and its application to olefin/paraffin separations. Chem. Commun. 2000, 1261–1262. [Google Scholar] [CrossRef]
- Liu, L.; Feng, X.; Chakma, A. Unusual behavior of poly(ethylene oxide)/AgBF4 polymer electrolyte membranes for olefin-paraffin separation. Sep. Purif. Technol. 2004, 38, 255–263. [Google Scholar] [CrossRef]
- Grande, C.A.; Araujo, J.D.P.; Cavenati, S.; Firpo, N.; Basaldella, E.; Rodrigues, A.E. New π-Complexation Adsorbents for Propane−Propylene Separation. Langmuir 2004, 20, 5291–5297. [Google Scholar] [CrossRef]
- Stoitsas, K.A.; Gotzias, A.; Kikkinides, E.S.; Steriotis, T.A.; Kanellopoulos, N.K.; Stoukides, M.; Zaspalis, V.T. Porous ceramic membranes for propane-propylene separation via the π-complexation mechanism: Unsupported systems. Microporous Mesoporous Mater. 2005, 78, 235–243. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, J.H.; Char, K.; Won, J.; Kang, Y.S. Nanocomposite silver polymer electrolytes as facilitated olefin transport membranes. J. Membr. Sci. 2006, 285, 102–107. [Google Scholar] [CrossRef]
- Kang, S.W.; Hong, J.; Char, K.; Kim, J.H.; Kim, J.; Kang, Y.S. Correlation between anions of ionic liquids and reduction of silver ions in facilitated olefin transport membranes. Desalination 2008, 233, 327–332. [Google Scholar] [CrossRef]
- Kang, S.W.; Kang, Y.S. Silver nanoparticles stabilized by crosslinked poly(vinyl pyrrolidone) and its application for facilitated olefin transport. J. Colloid Interface Sci. 2011, 353, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Fallanza, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Polymer-ionic liquid composite membranes for propane/propylene separation by facilitated transport. J. Membr. Sci. 2013, 444, 164–172. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Ortiz, I.; Li, K. Separation of Olefin Paraffin Gas Mixtures Using Ceramic Hollow Fiber Membrane Contactors. Ind. Eng. Chem. Res. 2013, 52, 7918–7929. [Google Scholar] [CrossRef]
- Fallanza, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Using membrane reactive absorption modeling to predict optimum process conditions in the separation of propane-propylene mixtures. Ind. Eng. Chem. Res. 2013, 52, 8843–8855. [Google Scholar] [CrossRef]
- Sun, H.; Ma, C.; Wang, T.; Xu, Y.; Yuan, B.; Kong, Y. Satellite TiO2nanoparticles induced by silver ion in polymer electrolytes membrane for propylene/propane separation. Mater. Chem. Phys. 2014, 148, 790–797. [Google Scholar] [CrossRef]
- Hamza, A.A.; Martin, J.; Perez, J.C. Continuous Olefin/Paraffin Separation with PermyleneTM Facilitated Transport Membranes from Imtex Membranes Corp. In Proceedings of the AIChE Spring Meeting and Global Congress on Process Safety, Austin, TX, USA, 29 April 2015. [Google Scholar]
- Park, C.H.; Lee, J.H.; Jung, J.P.; Kim, J.H. Mixed matrix membranes based on dual-functional MgO nanosheets for olefin/paraffin separation. J. Membr. Sci. 2017, 533, 48–56. [Google Scholar] [CrossRef]
- Kang, S.W.; Char, K.; Kim, J.H.; Kim, C.K.; Kang, Y.S. Control of ionic interactions in silver salt-polymer complexes with ionic liquids: Implications for facilitated olefin transport. Chem. Mater. 2006, 18, 1789–1794. [Google Scholar] [CrossRef]
- Chang, J.W.; Marrero, T.R.; Yasuda, H.K. Continuous process for propylene/propane separation by use of silver nitrate carrier and zirconia porous membrane. J. Membr. Sci. 2002, 205, 91–102. [Google Scholar] [CrossRef]
- Duan, S.; Ito, A.; Ohkawa, A. Separation of propylene/propane mixture by a supported liquid membrane containing triethylene glycol and a silver salt. J. Membr. Sci. 2003, 215, 53–60. [Google Scholar] [CrossRef]
- Chilukuri, P.; Rademakers, K.; Nymeijer, K.; Ham, L. Van Der Propylene/Propane Separation with a Gas/Liquid Membrane Contactor Using a Silver Salt Solution. Ind. Eng. Chem. Res. 2007, 8701–8709. [Google Scholar] [CrossRef]
- Takhtravanchi, M.; Kaghazchi, T.; Kargari, A. Immobilized Liquid Membrane for Propylene- Propane Separation. Proc. World Acad. Sci. Eng. Technol. 2008, 79–81. [Google Scholar]
- Kang, S.W.; Char, K.; Kang, Y.S. Novel Application of Partially Positively Charged Silver Nanoparticles for Facilitated Transport in Olefin/Paraffin Separation Membranes. Chem. Mater. 2008, 1308–1311. [Google Scholar] [CrossRef]
- Ortiz, A.; Ruiz, A.; Gorri, D.; Ortiz, I. Room temperature ionic liquid with silver salt as efficient reaction media for propylene/propane separation: Absorption equilibrium. Sep. Purif. Technol. 2008, 63, 311–318. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Kaghazchi, T.; Kargari, A.; Soleimani, M. A novel separation process for olefin gas purification: Effect of operating parameters on separation performance and process optimization. J. Taiwan Inst. Chem. Eng. 2009, 40, 511–517. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Separation of propylene-propane mixture using immobilized liquid membrane via facilitated transport mechanism. Sep. Sci. Technol. 2009, 44, 1198–1217. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Facilitated transport separation of propylene-propane: Experimental and modeling study. Chem. Eng. Process. Process Intensif. 2010, 49, 235–244. [Google Scholar] [CrossRef]
- Agel, F.; Pitsch, F.; Krull, F.F.; Schulz, P.; Wessling, M.; Melin, T.; Wasserscheid, P. Ionic liquid silver salt complexes for propene/propane separation. Phys. Chem. Chem. Phys. 2011, 13, 725–731. [Google Scholar] [CrossRef]
- Ortiz, A.; Marı, L.; Gorri, D.; De Haan, B.; Ortiz, I. Reactive Ionic Liquid Media for the Separation of Propylene/Propane Gaseous Mixtures. Ind. Eng. Chem. Res. 2010, 49, 7227–7233. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Kaghazchi, T.; Kargari, A. Supported liquid membrane separation of propylene-propane mixtures using a metal ion carrier. Desalination 2010, 250, 130–135. [Google Scholar] [CrossRef]
- Fallanza, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Experimental study of the separation of propane/propylene mixtures by supported ionic liquid membranes containing Ag+-RTILs as carrier. Sep. Purif. Technol. 2012, 97, 83–89. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Ortiz, I.; Li, K. Olefin/paraffin separation using ceramic hollow fiber membrane contactors. Procedia Eng. 2012, 44, 662–665. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, S.; Kamio, E.; Minami, R.; Matsuyama, H. A facilitated transport ion-gel membrane for propylene/propane separation using silver ion as a carrier. J. Membr. Sci. 2013, 431, 121–130. [Google Scholar] [CrossRef]
- Hong, G.H.; Ji, D.; Kang, S.W. Highly permeable ionic liquid/Cu composite membrane for olefin/paraffin separation. Chem. Eng. J. 2013, 230, 111–114. [Google Scholar] [CrossRef]
- Azizi, S.; Kaghazchi, T.; Kargari, A. Propylene/propane separation using N-methyl pyrrolidone/AgNO3 supported liquid membrane. J. Taiwan Inst. Chem. Eng. 2015, 57, 1–8. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Lee, K.B.; Won, J.; Kang, Y.S. Coordination structure of various ligands in crosslinked PVA to silver ions for facilitated olefin transport. Chem. Commun. 2002, 2, 2732–2733. [Google Scholar] [CrossRef]
- Zarca, R.; Ortiz, A.; Gorri, D.; Biegler, L.T.; Ortiz, I. Optimization of multistage olefin/paraffin membrane separation processes through rigorous modeling. AIChE J. 2019. [Google Scholar] [CrossRef]
- Bai, S.; Sridhar, S.; Khan, A.A. Metal-ion mediated separation of propylene from propane using PPO membranes. J. Membr. Sci. 1998, 147, 131–139. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ryu, H.; Bae, Y.; Kang, S.; Sik, H. Reactive polymer membranes containing cuprous complexes in olefin/paraffin separation. Chem. Commun. 2000, 3, 195–196. [Google Scholar] [CrossRef]
- Park, Y.S.; Won, J.; Kang, Y.S. Preparation of Poly ( ethylene glycol ) Brushes on Polysulfone Membranes for Olefin/Paraffin Separation. Macromolecules 2000, 16, 9662–9665. [Google Scholar] [CrossRef]
- Hong, S. Effect of water on the facilitated transport of olefins through solid polymer electrolyte membranes. J. Membr. Sci. 2001, 181, 289–293. [Google Scholar] [CrossRef]
- Sunderrajan, S.; Freeman, B.D.; Hall, C.K.; Pinnau, I. Propane and propylene sorption in solid polymer electrolytes based on poly(ethylene oxide) and silver salts. J. Membr. Sci. 2001, 182, 1–12. [Google Scholar] [CrossRef]
- Jose, B.; Ryu, J.H.; Kim, Y.J.; Kim, H.; Kang, Y.S.; Lee, S.D.; Kim, H.S. Effect of Plasticizers on the Formation of Silver Nanoparticles in Polymer Electrolyte Membranes for Olefin/Paraffin Separation. Chem. Mater. 2002, 14, 2134–2139. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Won, J.; Kang, Y.S. Anomalous temperature dependence of facilitated propylene transport in silver polymer electrolyte membranes. J. Membr. Sci. 2003, 227, 197–206. [Google Scholar] [CrossRef]
- Hun Park, H.; Won, J.; Oh, S.G.; Kang, Y.S. Effect of nonionic n-octyl β-D-glucopyranoside surfactant on the stability improvement of silver polymer electrolyte membranes for olefin/paraffin separation. J. Membr. Sci. 2003, 217, 285–293. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Won, J.; Kang, Y.S. Revelation of facilitated olefin transport through silver-polymer complex membranes using anion complexation. Macromolecules 2003, 36, 4577–4581. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.M.; Won, J.; Kang, Y.S. Dependence of facilitated olefin transport on the thickness of silver polymer electrolyte membranes. J. Membr. Sci. 2004, 236, 209–212. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, J.; Kang, Y.S. Olefin-induced dissolution of silver salts physically dispersed in inert polymers and their application to olefin/paraffin separation. J. Membr. Sci. 2004, 241, 403–407. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, J.; Kang, Y.S. Silver polymer electrolytes by π-complexation of silver ions with polymer containing C=C bond and their application to facilitated olefin transport membranes. J. Membr. Sci. 2004, 237, 199–202. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, J.H.; Won, J.; Char, K.; Kang, Y.S. Effect of amino acids in polymer/silver salt complex membranes on facilitated olefin transport. J. Membr. Sci. 2005, 248, 201–206. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, C.K.; Won, J.; Kang, Y.S. Role of anions for the reduction behavior of silver ions in polymer/silver salt complex membranes. J. Membr. Sci. 2005, 250, 207–214. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.M.; Won, J.; Kang, Y.S. Unusual separation property of propylene/propane mixtures through polymer/silver complex membranes containing mixed salts. J. Membr. Sci. 2005, 248, 171–176. [Google Scholar] [CrossRef]
- Hess, S.; Staudt-Bickel, C.; Lichtenthaler, R.N. Propene/propane separation with copolyimide membranes containing silver ions. J. Membr. Sci. 2006, 275, 52–60. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, Y.S.; Kim, J.H.; Kang, S.W. Selective coordination of silver ions to poly(styrene-b-(ethylene-co-butylene)-b-styrene) and its influence on morphology and facilitated olefin transport. Macromol. Res. 2008, 16, 676–681. [Google Scholar] [CrossRef]
- Mun, S.H.; Kang, S.W.; Cho, J.S.; Koh, S.K.; Kang, Y.S. Enhanced olefin carrier activity of clean surface silver nanoparticles for facilitated transport membranes. J. Membr. Sci. 2009, 332, 1–5. [Google Scholar] [CrossRef]
- Koh, J.H.; Kang, S.W.; Park, J.T.; Seo, J.A.; Kim, J.H.; Kang, Y.S. Synthesis of silver halide nanocomposites templated by amphiphilic graft copolymer and their use as olefin carrier for facilitated transport membranes. J. Membr. Sci. 2009, 339, 49–56. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Deng, M. Ultrathin solid polymer electrolyte PEI/Pebax2533/AgBF4 composite membrane for propylene/propane separation. Sep. Purif. Technol. 2011, 77, 46–52. [Google Scholar] [CrossRef]
- Pollo, L.D.; Duarte, L.T.; Anacleto, M.; Habert, A.C.; Borges, C.P. Polymeric membranes containing silver salts for propylene/propane separation. Brazilian J. Chem. Eng. 2012, 29, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Fallanza, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Effect of liquid flow on the separation of propylene/propane mixtures with a gas/liquid membrane contactor using Ag+-RTIL solutions. Desalin. Water Treat. 2011, 27, 123–129. [Google Scholar] [CrossRef]
- Najari, S.; Omidkhah, M.; Saeid, S. An Investigation on the Factors Affecting the Properties and Performance of Polymeric Nanocomposite Membranes for Olefin/Paraffin Separation. In Proceedings of the 5th International Conference on Ultrafine Grained and Nanostructured Materials, Tehran, Iran, 11–12 November 2015. [Google Scholar]
- Surya Murali, R.; Yamuna Rani, K.; Sankarshana, T.; Ismail, A.F.; Sridhar, S. Separation of Binary Mixtures of Propylene and Propane by Facilitated Transport through Silver Incorporated Poly(Ether-Block-Amide) Membranes. Oil Gas Sci. Technol. – Rev. d’IFP Energies Nouv. 2015, 70, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, S.; Koizumi, Y.; Loprete, K.; Pennisi, K.; Nemser, S.; Feiring, A.; Shangguan, N.; Murnen, H.; Lousenberg, D. Olefin-paraffin separation with customized amorphous fluoropolymer (CAF) facilitated transport membranes. In Proceedings of the 29th Ethylene Producers Conference 2017—Topical Conference at the 2017 AIChE Spring Meeting and 13th Global Congress on Process Safety, San Antonio, TX, USA, 28 March 2017. [Google Scholar]
- Jung, J.P.; Kim, M.J.; Bae, Y.S.; Kim, J.H. Facile preparation of Cu(I) impregnated MIL-101(Cr) and its use in a mixed matrix membrane for olefin/paraffin separation. J. Appl. Polym. Sci. 2018, 135, 46545. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Yang, J.S. Polymeric complex membranes for olefin/paraffin separation. In Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1996. [Google Scholar]
- Liu, J.; Chen, X.; Zhao, S.; Cao, X.; Shen, B. Multicycle Investigation of Normal Paraffin Separation from Naphtha To Improve Olefin and Aromatic Feed. Ind. Eng. Chem. Res. 2015, 54, 12664–12670. [Google Scholar] [CrossRef]
- Grande, C.A.; Lind, A.; Vistad, Ø.; Akporiaye, D. Olefin–Paraffin Separation Using Calcium-ETS-4. Ind. Eng. Chem. Res. 2014, 53, 15522–15530. [Google Scholar] [CrossRef]
- Son, S.J.; Choi, H.W.; Choi, D.K.; Lee, S.D.; Kim, H.S.; Kim, S.W. Selective absorption of isoprene from C5 mixtures by π complexation with Cu(I). Ind. Eng. Chem. Res. 2005, 44, 4717–4720. [Google Scholar] [CrossRef]
- Naghsh, M.; Sadeghi, M.; Moheb, A.; Chenar, M.P.; Mohagheghian, M. Separation of ethylene/ethane and propylene/propane by cellulose acetate-silica nanocomposite membranes. J. Membr. Sci. 2012, 423–424, 97–106. [Google Scholar] [CrossRef]
- Kanezashi, M.; Kawano, M.; Yoshioka, T.; Tsuru, T. Organic À Inorganic Hybrid Silica Membranes with Controlled Silica Network Size for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2012, 51, 944–953. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Koros, W.J. Matrimid® derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation. J. Membr. Sci. 2011, 380, 138–147. [Google Scholar] [CrossRef]
- Rungta, M.; Xu, L.; Koros, W.J. Carbon molecular sieve dense film membranes derived from Matrimid® for ethylene/ethane separation. Carbon 2012, 50, 1488–1502. [Google Scholar] [CrossRef]
- Motelica, A.; Bruinsma, O.S.L.; Kreiter, R.; den Exter, M.; Vente, J.F. Membrane Retrofit Option for Paraffin/Olefin Separation—A Technoeconomic Evaluation. Ind. Eng. Chem. Res. 2012, 51, 6977–6986. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Rungta, M.; Brayden, M.K.; Martinez, M.V.; Stears, B.A.; Barbay, G.A.; Koros, W.J. Olefins-selective asymmetric carbon molecular sieve hollow fiber membranes for hybrid membrane-distillation processes for olefin/paraffin separations. J. Membr. Sci. 2012, 423–424, 314–323. [Google Scholar] [CrossRef]
- Salinas, O.; Ma, X.; Litwiller, E.; Pinnau, I. High-performance carbon molecular sieve membranes for ethylene/ethane separation derived from an intrinsically microporous polyimide. J. Membr. Sci. 2016, 500, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Brayden, M. Impact of impurities on carbon molecular sieve membranes. In Proceedings of the AIChE Spring Meeting and Global Congress on Process Safety, San Antonip, TX, USA, 26–30 March 2017. [Google Scholar]
- Brayden, M.; Koros, W.J.; Xu, L.; Martinez, M.; Stears, B.A.; Barbay, G.A. Carbon Molecular Sieve Hollow Fiber Membranes for Olefin/Paraffin Separations. In Proceedings of the 2013 Spring Meeting and 9th Global Congress on Process Safety, San Antonio, TX, USA, 2 May 2013. [Google Scholar]
- Das, M. Membranes for Olefin/Paraffin Separations. Doctorate dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2009. [Google Scholar]
- Ma, X.; Lin, B.K.; Wei, X.; Kniep, J.; Lin, Y.S. Gamma-Alumina Supported Carbon Molecular Sieve Membrane for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2013, 52, 4297–4305. [Google Scholar] [CrossRef]
- Ma, X.; Williams, S.; Wei, X.; Kniep, J.; Lin, Y.S. Propylene/Propane Mixture Separation Characteristics and Stability of Carbon Molecular Sieve Membranes. Ind. Eng. Chem. Res. 2015, 54, 9824–9831. [Google Scholar] [CrossRef]
- Mei, L.; Wu, Y.; Zhou, X.; Yan, J.; Xu, F.; Li, Z. Adsorption performance of MIL-100(Fe) for separation of olefin–paraffin mixtures. J. Taiwan Inst. Chem. Eng. 2017, 70, 74–78. [Google Scholar] [CrossRef]
- Lin, Y.; Ji, W.; Wang, Y. Cuprous-Chloride-Modified Nanoporous Alumina Membranes for Ethylene−Ethane Separation. Ind. Eng. Chem. Res. 1999, 38, 2292–2298. [Google Scholar] [CrossRef]
- Van Miltenburg, A.; Zhu, W.; Kapteijn, F.; Moulijn, J.A. Adsorptive separation of light olefin/paraffin mixtures. Chem. Eng. Res. Des. 2006, 84, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Lin, C.C.H.; Kuznicki, T.M.; Hashisho, Z.; Kuznicki, S.M. Separation of a binary mixture of ethylene and ethane by adsorption on Na-ETS-10. Chem. Eng. Sci. 2010, 65, 3494–3498. [Google Scholar] [CrossRef]
- Aguado, S.; Bergeret, G.; Daniel, C.; Farrusseng, D. Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A. J. Am. Chem. Soc. 2012, 134, 14635–14637. [Google Scholar] [CrossRef]
- Hovestadt, M.; Friebe, S.; Helmich, L.; Lange, M.; Möllmer, J.; Gläser, R.; Mundstock, A.; Hartmann, M. Continuous Separation of light olefin/paraffin mixtures on ZIF-4 by pressure swing adsorption and membrane permeation. Molecules 2018, 23, 889. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Böhme, U.; Hovestadt, M.; Paula, C. Adsorptive Separation of Olefin/Paraffin Mixtures with ZIF-4. Langmuir 2015, 31, 12382–12389. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin Selective Ag-Exchanged X-Type Zeolite Membrane for Propylene/Propane and Ethylene/Ethane Separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Yancey, D.; Xu, L.; Martinez, M.; Brayden, M.; Koros, W. Iron-containing carbon molecular sieve membranes for advanced olefin/paraffin separations. J. Membr. Sci. 2018, 548, 609–620. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation Part A: Membrane preparation and characterization. J. Membr. Sci. 2013, 428, 445–453. [Google Scholar] [CrossRef]
- Pires, J.; Pinto, L.; Saini, V.K. Ethane Selective IRMOF-8 and Its Signi fi cance in Ethane-Ethylene Separation by Adsorption. ACS Appl. Mater. Interfaces 2014, 6, 12093–12099. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Bao, Z.; Ren, Q.; Deng, S.; Zhang, Z.; Su, B.; Xing, H.; Yang, Y. Fabrication of cuprous nanoparticles in MIL-101: An efficient adsorbent for the separation of olefin-paraffin mixtures. RSC Adv. 2014, 4, 20230–20233. [Google Scholar] [CrossRef]
- Luna-Triguero, A.; Vicent-Luna, J.M.; Becker, T.M.; Vlugt, T.J.H.; Dubbeldam, D.; Gómez-Álvarez, P.; Calero, S. Effective Model for Olefin/Paraffin Separation using (Co, Fe, Mn, Ni)-MOF-74. ChemistrySelect 2017, 2, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Böhme, U.; Barth, B.; Paula, C.; Kuhnt, A.; Schwieger, W.; Mundstock, A.; Caro, J.; Hartmann, M. Ethene/ethane and propene/propane separation via the olefin and paraffin selective metal-organic framework adsorbents CPO-27 and ZIF-8. Langmuir 2013, 29, 8592–8600. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Sholl, D.S. Screening of Copper Open Metal Site MOFs for Olefin/Paraffin Separations Using DFT-Derived Force Fields. J. Phys. Chem. C 2016, 120, 23044–23054. [Google Scholar] [CrossRef]
- Bendt, S.; Hovestadt, M.; Böhme, U.; Paula, C.; Döpken, M.; Hartmann, M.; Keil, F.J. Olefin/Paraffin Separation Potential of ZIF-9 and ZIF-71: A Combined Experimental and Theoretical Study. Eur. J. Inorg. Chem. 2016, 2016, 4440–4449. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, L.; Duan, Y.; Hou, Q.; Zhang, L.; Ding, L.X.; Xue, J.; Wang, H.; Caro, J. Paralyzed membrane: Current-driven synthesis of a metal-organic framework with sharpened propene/propane separation. Sci. Adv. 2018, 4, eaau1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari, M.; Chung, T.S. Natural gas purification and olefin/paraffin separation using thermal cross-linkable co-polyimide/ZIF-8 mixed matrix membranes. J. Membr. Sci. 2013, 444, 173–183. [Google Scholar] [CrossRef]
- Cadiau, A.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Eddaoudi, M. A metal-organic framework-based splitter for separating propylene from propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.K.; Kook Heon Char, S.; Sang Wook Kang, S.; Moon, S.-H. Facilitated olefin transporting polymer membrane containing metal nanoparticle. WO Patent 2007117087A1, 18 October 2007. [Google Scholar]
- Matsukata, M.; Seshimo, M.; Sakai, M.; Kimura, O.; Adachi, M. Olefin separation method and zeolite membrane complex. WO Patent 2015141686A1, 24 September 2015. [Google Scholar]
- Koros, W.J.; Xu, L.; Brayden, M.K.; Martinez, M.V.; Stears, B.A. A hollow fiber carbon molecular sieve membrane and preparation and use thereof. U.S. Patent 9,346,011, 24 May 2016. [Google Scholar]
- Long, J.R.; Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Bloch, E.; Murray, L. Metal-Organic Framework Adsorbents for Composite Gas Separation. U.S. Patent 13/965,098, 6 March 2014. [Google Scholar]
- Kim, H.S.; Kang, Y.S.; Lee, B.G.; Lee, H.J.; Ryu, J.H. Method for Producing Silver Salt-Containing Facilitated Transport Membrane for Olefin Separation Having Improved Stability. U.S. Patent 6,878,409, 12 April 2005. [Google Scholar]
- Kang, Y.S.; Char, K.H.; Kang, S.W. Silver Nanoparticle/Polymer Nanocomposite Membranes for Olefin Paraffin Separation and Method of Preparing the Same. U.S. Patent 7,491,262, 17 February 2009. [Google Scholar]
- Kang, Y.S.; Jung, B.; Kim, J.; Won, J.; Char, K.H.; Kang, S.W. Facilitated Transport Membranes for an Alkene Hydrocarbon Separation. U.S. Patent 11/011, 14 July 2005. [Google Scholar]
- Feiring, A.E.; Wilmington, D.; Lazzeri, J.; Ventura, C.; Majumdar, S.; Newark, D. Membrane Separation of Olefin and Paraffn Mixtures. U.S. Patent 20150025293A1, 22 January 2015. [Google Scholar]
- Liu, C.; Arlington Heights, I.; Liskey, C.W.; Tran, H.O.; Karns, N.K. High Selectivity Facilitated Transport Membranes and Their Use for Olefin/Paraffin Separation. U.S. Patent 20170354918A1, 14 December 2017. [Google Scholar]
- Liu, C.; Karns, N.K. Stable Facilitated Transport Membranes for Olefin/Paraffin Separations. U.S. Patent Application 10/258,929, 16 April 2019. [Google Scholar]
- Sheikholeslami, R. Fouling mitigation in membrane processes. Desal. J. 1999, 123, 45–53. [Google Scholar] [CrossRef]
- Van Zyl, A.J.; Kerres, J.A.; Cui, W.; Junginger, M. Application of new sulfonated ionomer membranes in the separation of pentene and pentane by facilitated transport. J. Membr. Sci. 1997, 137, 173–185. [Google Scholar] [CrossRef]
- Kovvali, A.S.; Chen, H.; Sirkar, K.K. Glycerol-based immobilized liquid membranes for olefin-paraffin separation. Ind. Eng. Chem. Res. 2002, 41, 347–356. [Google Scholar] [CrossRef]
- Jiang, B.; Tao, W.; Dou, H.; Sun, Y.; Xiao, X.; Zhang, L.; Yang, N. A Novel Supported Liquid Membrane Based on Binary Metal Chloride Deep Eutectic Solvents for Ethylene/Ethane Separation. Ind. Eng. Chem. Res. 2017, 56, 15153–15162. [Google Scholar] [CrossRef]
- Yang, J.S.; Hsiue, G.H. C4 olefin/paraffin separation by poly[(1-trimethylsilyl)-1-propyne]-graft-poly(acrylic acid)-Ag+ complex membranes. J. Membr. Sci. 1996, 111, 27–38. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, D.B.; Choi, D.K.; Ahn, B.S.; Kim, H.G.; Kim, H.S.; Lee, C.H.; Sung, J.Y. Highly selective facilitated transport membranes for isoprene/n-pentane separation. J. Membr. Sci. 2006, 279, 403–409. [Google Scholar] [CrossRef]
- Van Zyl, A.J.; Linkov, V.M. Influence of oxygen-containing hydrocarbons on the separation of olefin/paraffin mixtures using facilitated transport. J. Membr. Sci. 1997, 133, 15–26. [Google Scholar] [CrossRef]
- Ma, L.; Svec, F.; Tan, T.; Lv, Y. Mixed Matrix Membrane Based on Cross-Linked Poly[(ethylene glycol) methacrylate] and Metal–Organic Framework for Efficient Separation of Carbon Dioxide and Methane. ACS Appl. Nano Mater. 2018, 1, 2808–2818. [Google Scholar] [CrossRef]
- Chai, S.; Du, H.; Zhao, Y.; Lin, Y.; Kong, C.; Chen, L. Fabrication of highly selective organosilica membrane for gas separation by mixing bis(triethoxysilyl)ethane with methyltriethoxysilane. Sep. Purif. Technol. 2019, 222, 162–167. [Google Scholar] [CrossRef]
- Sun, Q.; Qi, B.; Liu, A.; Guo, X.; Zhang, J. Separation of H2/CH4 Through TBAB Hydrate Membrane. Int. J. New Technol. Sci. Eng. 2015, 2, 39–46. [Google Scholar]
- Bandehali, S.; Kargari, A.; Moghadassi, A.; Saneepur, H.; Ghanbari, D. Acrylonitrile-butadiene-styrene/poly(vinyl acetate)/nanosilica mixed matrix membrane for He/CH4 separation. Asia-Pacific J. Chem. Eng. 2014, 9, 638–644. [Google Scholar] [CrossRef]
- Lei, G.; Liu, C.; Xie, H.; Song, F. Separation of the hydrogen sulfide and methane mixture by the porous graphene membrane: Effect of the charges. Chem. Phys. Lett. 2014, 599, 127–132. [Google Scholar] [CrossRef]
- Sklari, S.D.; Zaspalis, V.T. A novel system of Al100P60Ozmicroporous ceramic membrane for hydrogen separation from hydrogen/propane mixtures. Microporous Mesoporous Mater. 2007, 99, 176–180. [Google Scholar] [CrossRef]
- Bux, H.; Feldhoff, A.; Cravillon, J.; Wiebcke, M.; Li, Y.S.; Caro, J. Oriented zeolitic imidazolate framework-8 membrane with sharp H 2/C3H8 molecular sieve separation. Chem. Mater. 2011, 23, 2262–2269. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Shahidi, K.; Mohammadi, T. Preparation and C3H8/gas separation properties of a synthesized single layer PDMS membrane. Sep. Sci. Technol. 2010, 45, 592–603. [Google Scholar] [CrossRef]
- Chen, J.; Eldridge, R.B.; Rosen, E.L.; Bielawski, C.W. A study of Cu(I)-ethylene complexation for olefin-paraffin separation. AIChE J. 2011, 57, 630–644. [Google Scholar] [CrossRef]
- Da Costa, A.R.; Wijmans, H.; Baker, R.W. Ethylene Recovery by Membrane Technology. Proceedings of the 13th Ethylene Producers’ Conference, Houston, TX. 2001, pp. 78–87. Available online: https://www.tib.eu/en/search/id/BLCP%3ACN046215552/Ethylene-Recovery-by-Membrane-Technology/ (accessed on 7 November 2019).
- Yu, L.; Grahn, M.; Ye, P.; Hedlund, J. Ultra-thin MFI membranes for olefin/nitrogen separation. J. Membr. Sci. 2017, 524, 428–435. [Google Scholar] [CrossRef]
- Ho, W.S.; Dalrymple, D.C. Facilitated transport of olefins in Ag+-containing polymer membranes. J. Membr. Sci. 1994, 91, 13–25. [Google Scholar] [CrossRef]
- Funke, H.H.; Noble, R.D.; Koval, C.A. Separation of gaseous olefin isomers using facilitated transport membranes. J. Membr. Sci. 1993, 82, 229–236. [Google Scholar] [CrossRef]
- Goering, R.M.; Bowman, C.N.; Koval, C.A.; Noble, R.D.; Williamson, D.L. Role of ion-exchange membrane morphology and sorption properties in facilitated transport di-olefin/mono-olefin separations. J. Membr. Sci. 1998, 144, 133–143. [Google Scholar] [CrossRef]
- Yave, W.; Shishatskiy, S.; Abetz, V.; Matson, S.; Litvinova, E.; Khotimskiy, V.; Peinemann, K.V. A novel poly(4-methyl-2-pentyne)/TiO2 hybrid nanocomposite membrane for natural gas conditioning: Butane/methane separation. Macromol. Chem. Phys. 2007, 208, 2412–2418. [Google Scholar] [CrossRef] [Green Version]
- Hrabánek, P.; Zikánová, A.; Bernauer, B.; Fíla, V.; Kočiřík, M. A route to MFI zeolite-α-alumina composite membranes for separation of light paraffins. Desalination 2008, 224, 76–80. [Google Scholar] [CrossRef]
- Jung, S.; Palgunadi, J.; Kim, J.H.; Lee, H.; Ahn, B.S.; Cheong, M.; Kim, H.S. Highly efficient metal-free membranes for the separation of acetylene/olefin mixtures: Pyrrolidinium-based ionic liquids as acetylene transport carriers. J. Membr. Sci. 2010, 354, 63–67. [Google Scholar] [CrossRef]
- Ni, H.; Hsu, C.S.; Ma, C.; Shi, Q.; Xu, C. Separation and Characterization of Olefin/Paraffin in Coal Tar and Petroleum Coker Oil. Energy Fuels 2013, 27, 5069–5075. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, W.; Jacquemin, J.; Goodrich, P.; Atilhan, M.; Khraisheh, M.; Rooney, D.; Thompson, J. Enhancing Liquid-Phase Ole fi n—Para ffi n Separations Using Novel Silver-Based Ionic Liquids. J. Chem. Eng. Data 2015, 60, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Shokrian, M.; Sadrzadeh, M.; Mohammadi, T. Neural Network Modelling of C3H8 Separation from CH4 and H2 Using PDMS Membrane; Iran University of Science and Technology: Tehran, Iran. Available online: https://www.academia.edu/15943093/NEURAL_NETWORK_MODELLING_OF_C3H8_SEPARATION_FROM_CH4_and_H2_USING_PDMS_MEMBRANE (accessed on 7 November 2019).
- Yang, J.; Hsiue, G. Novel dry poly [(1-trimethylsilyl)-1-propyne]-AgC10 4 complex membranes for olefin/paraffin separations. Science 1996, 120, 69–76. [Google Scholar]
- Okamoto, K.; Noborio, K.; Hao, J.; Tanaka, K.; Kita, H. Permeation and separation properties of polyimide membranes to 1,3-butadiene and n-butane. J. Membr. Sci. 1997, 134, 171–179. [Google Scholar] [CrossRef]
- Wang, Y.; Thompson, J.; Zhou, J.; Goodrich, P.; Atilhan, M.; Pensado, A.S.; Kirchner, B.; Rooney, D.; Jacquemin, J.; Khraisheh, M. Use of water in aiding olefin/paraffin (liquid + liquid) extraction via complexation with a silver bis(trifluoromethylsulfonyl)imide salt. J. Chem. Thermodyn. 2014, 77, 230–240. [Google Scholar] [CrossRef]
- Bessarabov, D.G.; Theron, J.P.; Sanderson, R.D.; Schwarz, H.H.; Schossig-Tiedemann, M.; Paul, D. Separation of 1-hexene/n-hexane mixtures using a hybrid membrane/extraction system. Sep. Purif. Technol. 1999, 16, 167–174. [Google Scholar] [CrossRef]
- Hsiue, G.; Yang, J. Ag+ contained complex membrane for the separation of C4 olefin/paraffin mixture. J. Polym. Res. 1994, 1, 35–41. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, X.; Ruan, H.; Wu, L.; Qiu, J.; Gao, C. Synthesis of AgCl/PMMA hybrid membranes and their sorption performance of cyclohexane/cyclohexene. J. Membr. Sci. 2007, 304, 118–124. [Google Scholar] [CrossRef]
- Sungpet, A.; Way, J.D.; Koval, C.A.; Eberhart, M.E. Silver doped Nafion-poly(pyrrole) membranes for facilitated permeation of liquid-phase olefins. J. Membr. Sci. 2001, 189, 271–279. [Google Scholar] [CrossRef]
- Rege, S.U.; Padin, J.; Yang, R.T. Olefin/Paraffin Separations by Adsorption: π-Complexation vs. Kinetic Separation. AIChE J. 1998, 44, 799–809. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Jiang, C.; Xu, Y.; Wang, M.; Niu, Q.J. Molecular simulation for separation of ethylene and ethane by functionalised graphene membrane. Mol. Simul. 2019, 45, 1322–1331. [Google Scholar] [CrossRef]
- Chan, S.S.; Wang, R.; Chung, T.-S.; Liu, Y. C2 and C3 hydrocarbon separations in poly(1,5-naphthalene-2,2′-bis(3,4-phthalic) hexafluoropropane) diimide (6FDA-1,5-NDA) dense membranes. J. Membr. Sci. 2002, 210, 55–64. [Google Scholar] [CrossRef]
- Ilinich, O.M.; Zamaraev, K.I. Separation of ethylene and ethane over polyphenyleneoxides membranes: transient increase of selectivity. J. Membr. Sci. 1993, 82, 141–147. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Chan, S.S.; Chung, T.S.; Liu, Y.; Wang, R. Gas and hydrocarbon (C2and C3) transport properties of co-polyimides synthesized from 6FDA and 1,5-NDA (naphthalene)/Durene diamines. J. Membr. Sci. 2003, 218, 235–245. [Google Scholar] [CrossRef]
- Huang, J.-F.; Luo, H.; Liang, C.; Jiang, D.; Dai, S. Advanced Liquid Membranes Based on Novel Ionic Liquids for Selective Separation of Olefin/Paraffin via Olefin-Facilitated Transport. Ind. Eng. Chem. Res. 2008, 47, 881–888. [Google Scholar] [CrossRef]
- Luna-Triguero, A.; Vicent-Luna, J.M.; Gómez-Álvarez, P.; Calero, S. Olefin/Paraffin Separation in Open Metal Site Cu-BTC Metal-Organic Framework. J. Phys. Chem. C 2017, 121, 3126–3132. [Google Scholar] [CrossRef]
- Yang, J.S.; Hsiue, G.H. Swollen polymeric complex membranes for olefin/paraffin separation. J. Membr. Sci. 1998, 138, 203–211. [Google Scholar] [CrossRef]
- Yang, J.S.; Hsiue, G.H. Selective olefin permeation through Ag(I) contained silicone rubber-graft-poly(acrylic acid) membranes. J. Membr. Sci. 1997, 126, 139–149. [Google Scholar] [CrossRef]
- Won, J.; Dong, B.K.; Yong, S.K.; Dai, K.C.; Hoon, S.K.; Chan, K.K.; Chang, K.K. An ab initio study of ionic liquid silver complexes as carriers in facilitated olefin transport membranes. J. Membr. Sci. 2005, 260, 37–44. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.B.; Choi, D.K.; Lee, H.; Kim, H.S.; Won, J. Isoprene/pentane separation using facilitated transport membranes. J. Membr. Sci. 2004, 233, 113–117. [Google Scholar] [CrossRef]
- Jiang, C.; Hou, Y.; Wang, N.; Li, L.; Lin, L.; Niu, Q.J. Propylene/propane separation by porous graphene membrane: Molecular dynamic simulation and first-principle calculation. J. Taiwan Inst. Chem. Eng. 2017, 78, 477–484. [Google Scholar] [CrossRef]
- Kanezashi, M.; Shazwani, W.N.; Yoshioka, T.; Tsuru, T. Separation of propylene/propane binary mixtures by bis(triethoxysilyl) methane (BTESM)-derived silica membranes fabricated at different calcination temperatures. J. Membr. Sci. 2012, 415–416, 478–485. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.; Shin, J.W.; Tak, K.; Park, Y.K. Performance Study of multistage membrane and hybrid distillation processes for propylene/propane separation. Can. J. Chem. Eng. 2017, 95, 2390–2397. [Google Scholar] [CrossRef]
- Amedi, H.R.; Aghajani, M. Modified zeolitic–midazolate framework 8/poly(ether-block-amide) mixed-matrix membrane for propylene and propane separation. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Liao, K.S.; Lai, J.Y.; Chung, T.S. Metal ion modified PIM-1 and its application for propylene/propane separation. J. Membr. Sci. 2016, 515, 36–44. [Google Scholar] [CrossRef]
- Lee, K.R.; Hwang, S.T. Separation of propylene and propane by polyimide hollow-fiber membrane module. J. Membr. Sci. 1992, 73, 37–45. [Google Scholar] [CrossRef]
- Tanaka, K.; Taguchi, A.; Hao, J.; Kita, H.; Okamoto, K. Permeation and separation properties of polyimide membranes to olefins and paraffins. J. Membr. Sci. 1996, 121, 197–207. [Google Scholar] [CrossRef]
- Sridhar, S.; Khan, A.A. Simulation studies for the separation of propylene and propane by ethylcellulose membrane. J. Membr. Sci. 1999, 159, 209–219. [Google Scholar] [CrossRef]
- Krol, J.J.; Boerrigter, M.; Koops, G.H. Polyimide hollow fiber gas separation membranes: Preparation and the suppression of plasticization in propane/propylene environments. J. Membr. Sci. 2001, 184, 275–286. [Google Scholar] [CrossRef]
- Yoshino, M.; Nakamura, S.; Kita, H.; Okamoto, K.I.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation performance of asymmetric hollow fiber membrane of 6FDA/BPDA-DDBT copolyimide. J. Membr. Sci. 2003, 212, 13–27. [Google Scholar] [CrossRef]
- Semenova, S.I. Polymer membranes for hydrocarbon separation and removal. J. Membr. Sci. 2004, 231, 189–207. [Google Scholar] [CrossRef]
- Chng, M.L.; Xiao, Y.; Chung, T.S.; Toriida, M.; Tamai, S. Enhanced propylene/propane separation by carbonaceous membrane derived from poly (aryl ether ketone)/2,6-bis(4-azidobenzylidene)-4-methyl-cyclohexanone interpenetrating network. Carbon 2009, 47, 1857–1866. [Google Scholar] [CrossRef]
- Das, M.; Koros, W.J. Performance of 6FDA-6FpDA polyimide for propylene/propane separations. J. Membr. Sci. 2010, 365, 399–408. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations. J. Membr. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- Askari, M.; Xiao, Y.; Li, P.; Chung, T.S. Natural gas purification and olefin/paraffin separation using cross-linkable 6FDA-Durene/DABA co-polyimides grafted with α, β, and γ-cyclodextrin. J. Membr. Sci. 2012, 390–391, 141–151. [Google Scholar] [CrossRef]
- Yang, D.; Le, L.; Martinez, R.; Morrison, M. Hollow fibers structured packings in olefin/paraffin distillation: Apparatus scale-up and long-term stability. Ind. Eng. Chem. Res. 2013, 52, 9165–9179. [Google Scholar] [CrossRef]
- Swaidan, R.J.; Ma, X.; Litwiller, E.; Pinnau, I. Enhanced propylene/propane separation by thermal annealing of an intrinsically microporous hydroxyl-functionalized polyimide membrane. J. Membr. Sci. 2015, 495, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Najari, S.; Hosseini, S.S.; Omidkhah, M.; Tan, N.R. Phenomenological modeling and analysis of gas transport in polyimide membranes for propylene/propane separation. RSC Adv. 2015, 5, 47199–47215. [Google Scholar] [CrossRef]
- Sun, H.-X.; Yuan, B.-B.; Li, P.; Wang, T.; Xu, Y.-Y. Preparation of nanoporous graphene and the application of its nanocomposite membrane in propylene/propane separation. Funct. Mater. Lett. 2015, 08, 1550019. [Google Scholar] [CrossRef]
- Visser, T.; Wessling, M. Auto and mutual plasticization in single and mixed gas C3transport through Matrimid-based hollow fiber membranes. J. Membr. Sci. 2008, 312, 84–96. [Google Scholar] [CrossRef]
- Giannakopoulos, I.G.; Nikolakis, V. Separation of Propylene/Propane Mixtures Using Faujasite-Type Zeolite Membranes. Ind. Eng. Chem. Res. 2005, 44, 226–230. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Reyes, S.C. Adsorptive separation of light olefins from paraffins. Microporous Mesoporous Mater. 2007, 104, 59–66. [Google Scholar] [CrossRef]
- Gascon, J.; Blom, W.; van Miltenburg, A.; Ferreira, A.; Berger, R.; Kapteijn, F. Accelerated synthesis of all-silica DD3R and its performance in the separation of propylene/propane mixtures. Microporous Mesoporous Mater. 2008, 115, 585–593. [Google Scholar] [CrossRef]
- Pan, Y.; Li, T.; Lestari, G.; Lai, Z. Effective separation of propylene/propane binary mixtures by ZIF-8 membranes. J. Membr. Sci. 2012, 390–391, 93–98. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.; Ma, C.; Rownaghi, A.; Jones, C.W.; Nair, S. ZIF-8 Membranes via Interfacial Microfluidic Processing in Polymeric Hollow Fibers: Efficient Propylene Separation at Elevated Pressures. ACS Appl. Mater. Interfaces 2016, 8, 25337–25342. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Xiang, L.; Xu, Y.; Pan, Y. Enhanced C3H6/C3H8 separation performance in poly(vinyl acetate) membrane blended with ZIF-8 nanocrystals. Chem. Eng. Sci. 2018, 179, 1–12. [Google Scholar] [CrossRef]
- Shrestha, S.; Dutta, P.K. Modification of a continuous zeolite membrane grown within porous polyethersulfone with Ag(I) cations for enhanced propylene/propane gas separation. Microporous Mesoporous Mater. 2019, 279, 178–185. [Google Scholar] [CrossRef]
- Maghsoudi, H. Theoretical screening zeolites for membrane separation of propylene/propane mixtures. Polyolefins J. 2017, 5, 1–14. [Google Scholar]














| Membrane Type | Definition and Characteristics | Drawbacks |
|---|---|---|
| CMS | Carbon molecular sieves constitute a class of amorphous carbon materials produced through the pyrolysis of microporous polymer precursors [8,59,62]. Although the surface area is relatively small, the characteristic pore sizes are small with narrow size distributions, enabling the separation at molecular level based on the size and shape of the molecules [8,63]. | The pore diameters can be significantly different from characteristic sizes of molecules that must be separated. CMS materials can be fragile and it may be difficult to scale-up the production process [59,64]. |
| Polymer | Polymer membranes can be casted with different thicknesses and porosities (PIMs) [2,58]. Carriers can be easily added to allow for; facilitated transport [8,65]. | Polymer films can present low gas permeabilities and selectivities [8] and are subject to swelling, plasticization, and heterogeneous structure and porosity [66]. Carriers can be subject to deactivation by poisonous agents [59]. |
| Zeolite | Zeolites are hydrated aluminosilicate materials, which possess outstanding ion-exchange and sorption properties [8,67]. Separation is based on pore sizes and polarity, which can be uniform [66] and are controllable [8]. Zeolites present higher thermal and chemical stabilities than polymers, large surface areas, high selectivities and high permeabilities [8,68] | Preparation conditions can be aggressive, with combination of high temperatures, high pressures and extreme pH values. The ranges of pore sizes can be narrow, adhesion properties onto different substrates can be poor and the production costs can be high [66,69]. |
| MOF | Metal organic frameworks are hybrid materials constituted by metallic nodes, which are linked to each other through organic bridges, leading to functional porous structures [66,70]. | The manufacture of continuous MOF layers can be difficult and the produced films can be very fragile. Adhesion properties onto different substrates can be poor and the production costs can be high [66]. |
| MMM | Mixed matrix membranes are hybrid materials produced through mixing of polymers and inorganic fillers, including activated carbon, carbon nanotubes, zeolites, silica, molecular sieves, and MOFs [66]. Consequently, the final membrane properties can be manipulated with high flexibility. | The matrix and fillers must be compatible and filler aggregation and sedimentation must be prevented during membrane preparation [8]. |
| Separation Mechanisms | Membrane Material | Permeation Mechanisms | Drawbacks |
|---|---|---|---|
| Solution-diffusion | Polymers | (1) Molecules adsorb and dissolve into the membrane material. (2) Molecules diffuse through the membrane, driven by pressure, temperature or concentration gradients. (3) Molecules desorb into the bulk stream in the permeate side [8]. | Gas solubility in conventional polymer membranes is closely related to compressibility [8]. Discrimination of olefin/paraffin pairs is not effective [59]. |
| Interaction between olefin and membrane | Zeolites, polymers, MOF, MMM, ionic liquids, adsorbents, absorbents | (1) Carriers can form complexes with gaseous components and allow the facilitated transport [59]. (2) The adsorption step can be followed by stepwise thermal regeneration and desorption [1]. | Carriers are subject to deactivation by poisonous agents [59] and can be very expensive [71]. |
| Molecular sieving | MOF, CMS, zeolites | (1) Molecules are separated due to different molecular sizes and shapes (geometrical selectivity) [8]. | The pore diameters can be significantly different from characteristic sizes of molecules that must be separated. It may be difficult to scale-up the production process [59]. |
| # | Category | # | Category | # | Category |
|---|---|---|---|---|---|
| 1 | Institution | 6 | Feed composition | 11 | Separated gases |
| 2 | Country | 7 | Selectivity or separation factor | 12 | Type of material |
| 3 | Journal | 8 | Permeability | 13 | Metal carrier |
| 4 | Year of publication | 9 | Operation temperature/°C | 14 | Layout |
| 5 | Number of citations | 10 | Operation pressure/bar | 15 | Lifetime |
| Ranking | Journal | IF | NP | Percentage (%) |
|---|---|---|---|---|
| 1 | Journal of Membrane Science | 6.03 | 101 | 34% |
| 2 | Industrial and Engineering Chemistry Research | 2.84 | 32 | 11% |
| 3 | Separation and Purification Technology | 3.35 | 14 | 5% |
| 4 | Microporous and Mesoporous Materials | 3.61 | 5 | 2% |
| 5 | Journal of the American Chemical Society | 13.85 | 5 | 2% |
| 6 | Chemical Communications | 6.31 | 5 | 2% |
| 7 | Separation Science and Technology | 1.10 | 5 | 2% |
| 8 | Chemical Engineering Science | 2.89 | 5 | 2% |
| Ranking | Country | Total Publications | Percentage (%) |
|---|---|---|---|
| 1 | USA | 63 | 21% |
| 2 | South Korea | 46 | 16% |
| 3 | Iran | 27 | 9% |
| 4 | China | 26 | 9% |
| 5 | Japan | 20 | 7% |
| 6 | Netherlands | 18 | 6% |
| 7 | Malaysia | 13 | 5% |
| 8 | Germany | 12 | 4% |
| 9 | Spain | 10 | 4% |
| Ranking | Country | Percentage (%) |
|---|---|---|
| 1 | USA | 56 |
| 2 | Korea | 21 |
| 3 | France | 12 |
| Ranking | Institutions | Documents | Percentage (%) |
|---|---|---|---|
| 1 | Korea Institute of Science and Technology (South Korea) | 21 | 7 |
| 2 | Amirkabir University of Technology (Iran) | 12 | 4 |
| 2 | Georgia Institute of Technology (USA) | 12 | 4 |
| 3 | University of Twente (The Nerthelands) | 10 | 3 |
| Ranking | Institution | Countries | Percentage (%) |
|---|---|---|---|
| 1 | ExxonMobil Research and Engineering Company | USA | 9 |
| 1 | UOP LLC | USA | 9 |
| 1 | Institut Français du Petrole | France | 9 |
| 1 | Industry-University Cooperation Foundation Hanyang University | Korea | 9 |
| 1 | Korea Institute of Science and Technology | Korea | 9 |
| 2 | Membrane Technology and Research, Inc. | USA | 6 |
| Ranking | Paper | Separated Gases | Type of Membrane (Name) | Citations | Ref. |
|---|---|---|---|---|---|
| 1 | Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites Authors: Bloch, E.D., Queen, W.L., Krishna, R., Zadrozny, J.M., Brown, C.M., Long, J.R. Source: Science (2012) | Ethylene/Ethane Propane/Propane | MOF (Fe2(dobdc)) | 1008 | [101] |
| 2 | Pushing the limits on possibilities for large scale gas separation: which strategies? Authors: Koros, W.J., Mahajan, R. Source: Journal of Membrane Science (2000) | Olefin/Paraffin Others | Various (Review) | 829 | [102] |
| 3 | Gas solubility, diffusivity and permeability in poly(ethylene oxide) Authors: Lin, H., Freeman, B.D. Source: Journal of Membrane Science (2004) | Ethylene/Ethane Propylene/Propane Others | Polymer (PEO) | 627 | [103] |
| 4 | Olefin/Paraffin Separation Technology: A Review Author: Eldridge, R.B. Source: Industrial and Engineering Chemistry Research (1993) | Olefin/Paraffin | Various (Review) | 580 | [1] |
| 5 | Application of membrane separation processes in petrochemical industry: a review Authors: Ravanchi, M.T., Kaghazchi, T., Kargari, A. Source: Desalination (2009) | Propylene/Propane | Polymer (6FDA-DDBT) | 487 | [53] |
| 6 | Title: Zeolitic Imidazolate Frameworks for Kinetic Separation of Propane and Propene Author(s): Li, K., Olson, D.H., Seidel, J., Emge, T.J., Gong, H., Zeng, H., Li, J. Source: Journal of the American Chemical Society (2009) | Propylene/Propane | MOF (ZIF-8) | 466 | [104] |
| 7 | Title: Ethane/Ethene Separation Turned on Its Head: Selective Ethane Adsorption on the Metal-Organic Framework ZIF-7 through a Gate-Opening Mechanism. Author(s): Gücüyener, C., Bergh, J.V.D., Gascon, J., Kapteijn, F. Source: Journal of the American Chemical Society (2010) | Ethylene/Ethane | MOF (ZIF-7) | 408 | [77] |
| 8 | Title: Olefin/Paraffin Separations by Reactive Absorption: A Review Author(s): Safarik, D.J., Eldridge, R.B. Source: Industrial and Engineering Chemistry Research (1998) | Olefin/Paraffin | Absorbent (Review) | 312 | [105] |
| Separated Gases | Type of Material | Name of the Material | Carrier | Selectivity or Sep Factor | Ref. |
|---|---|---|---|---|---|
| ethylene/ethane | FT/Hybrid | not specified | Ag | NS | [125] |
| ethylene/ethane | FT/Hybrid | not specified | Ag+ | SF 65 ethylene/ethane | [126] |
| ethylene/ethane | FT/Hybrid | Chitosan/Ag (Imtex) | Ag+ | SF 100 ethylene/ethane | [127] |
| ethylene/ethane | FT/Hybrid | 5A zeolite | Ag+ | S 27.4 ethylene | [128] |
| ethylene/ethane | FT/Hybrid | Fe2(dobdc) | Ag+ | S 13.6 ethylene | [128] |
| ethylene/ethane | FT/Liquid | Fluoropore FP-010/AgNO3 (Sumitomo) | Ag+ | SF 460 ethylene/ethane | [90] |
| ethylene/ethane | FT/Liquid | polysulfone | Ag+ | SF 420 ethylene/ethane | [129] |
| ethylene/ethane | FT/Liquid | PEO/PBT/AgNO3 | Ag+ | SF 165 ethylene/ethane | [130] |
| ethylene/ethane | FT/Liquid | EPDM-SPEEK | Ag+ | SF 2700 ethylene/ethane | [131] |
| ethylene/ethane | FT/Liquid | [4-mebupy]BF4 | Ag+ | S 3 ethylene | [132] |
| ethylene/ethane | FT/Liquid | Cu SILM supported PVDF | Cu | S 11.8 | [133] |
| ethylene/ethane | FT/Liquid | PIL/40IL-Ag+ 1.25 M | Ag+ | S 7.24 etylene | [134] |
| ethylene/ethane | FT/Liquid | ZnCl2/[BMIM][Cl] | Zn IL | S 178 | [135] |
| ethylene/ethane | FT/Liquid | CuCl/ChCl-EG-based SLMs | Cu | S 12.5 | [136] |
| ethylene/ethane | FT/Liquid | CuCl/DESs-SLMs | IL | SF 20 ethylene/ethane | [135] |
| ethylene/ethane | FT/Liquid | DESs-SLMs | Ag+ | S 50 -100 ethylene | [111] |
| ethylene/ethane | FT/Polymer | Nafion N-117 | Ag+ | SF 540 ethylene/ethane | [137] |
| ethylene/ethane | FT/Polymer | AgBF4/PVP | Ag+ | SF 2.3 ethylene/ethane | [138] |
| ethylene/ethane | FT/Polymer | AgBF4/PEO | Ag+ | SF 240 ethylene/ethane | [139] |
| ethylene/ethane | FT/Polymer | Pebax® 4011 and Pebax® 2533 (Atofina) | Ag+ | NS | [140] |
| ethylene/ethane | FT/Polymer | AgNO3/polyethersulfone (Daicel) | Ag+ | SF 1100 ethylene/ethane | [141] |
| ethylene/ethane | FT/Polymer | PA 1 2-PTMO/AgBF4 | Ag+ | SF 20 ethylene/ethane | [142] |
| ethylene/Ethane | FT/Polymer | POZ/AgBF4 | Ag+ | SF 5 ethylene/ethane | [143] |
| ethylene/ethane | FT/Polymer | EPDM | Ag+ | SF 72.5 ethylene/ethene | [136] |
| ethylene/ethane | FT/Polymer | AgNO3/polyethersulfone (Daicel) | Ag+ | SF 374 ethylene/ethane | [144] |
| ethylene/ethane | FT/Polymer | PebaxTM 2533/AgBF4 | Ag+ | NS | [145] |
| ethylene/ethane | FT/Polymer | 3c | Ag+ | SF 115 ethylene/ethane | [146] |
| ethylene/ethane | FT/Polymer | SiO2 Poly(sodium acrylate) Ag+ | Ag+ | SF 94 ethylene/ethane | [147] |
| ethylene/ethane | FT/Polymer | Pebax® 2533/AgBF4 (Arkema) | Ag+ | SF 55 ethylene/ethane | [115] |
| ethylene/ethane | FT/Polymer | 28% PVDF/72% triacetin/AgNO3 | Ag+ | NS | [129] |
| ethylene/ethane | FT/Polymer | Psf/AgNO3 | Ag+ | NS | [148] |
| ethylene/ethane | FT/Polymer | PSf/PTMSP | Ag+ | NS | [149] |
| ethylene/ethane | FT/Polymer | AgBF4-PVMK membrane | Ag+ | ethylene/ethane | [150] |
| ethylene/ethane | FT/Polymer | PEO-AgBF4 | Ag+ | NS | [151] |
| propylene/propane | FT/Hybrid | Ag/SBA-15 | Ag+ | S 10 propylene | [152] |
| propylene/propane | FT/Hybrid | Ag/c-Al2O3 | Ag+ | S 1.2 propane | [153] |
| propylene/propane | FT/Hybrid | POZ/AgNO3/SiO2 (fumed silica nanoparticles) (1:1:0.1) | Ag+ | S 90.0 propylene/propane | [154] |
| propylene/propane | FT/Hybrid | POZ/AgNO3/BMIM+NO3− | Ag+ | S 32.0 propylene/propane | [155] |
| propylene/propane | FT/Hybrid | POZ/AgNO3/BMIM+BF4− | Ag+ | S 31.8 propylene/propane | [155] |
| propylene/propane | FT/Hybrid | POZ/AgNO3/BMIM+CF3SO3− | Ag+ | S 33.2 propylene/propane | [155] |
| propylene/propane | FT/Hybrid | PVP/Nano Au (Seahan) | Au | S 22 propylene | [96] |
| propylene/propane | FT/Hybrid | POZ | Ag+ | SF 20–22.5 propylene/propane | [156] |
| propylene/propane | FT/Hybrid | PVDF-HFP/BMImBF4−Ag+ | Ag+ | S 700 propane | [157] |
| propylene/propane | FT/Hybrid | AgNO3/Al2O3 | Ag+ | NS | [158] |
| propylene/propane | FT/Hybrid | MICRODYN MD020 TP 2N | Ag+ | NS | [159] |
| propylene/propane | FT/Hybrid | TiO2-PEO-AgBF4 | Ag+ | S 19 propylene/propane | [160] |
| propylene/propane | FT/Hybrid | Permylene (Imtex) | Ag+ | NS | [161] |
| propylene/propane | FT/Hybrid | PHMEP-g-PEGBEM/AgBF4/MgO-NS | Ag+ | SF 12.9 propylene/ propane | [162] |
| propylene/propane | FT/Liquid | POZ/AgNO3/BMIM+BF4− | Ag+ | SF 31.8 propylene/propane | [163] |
| propylene/propane | FT/Liquid | POZ/AgNO3/BMIM+NO3− | Ag+ | SF 32 propylene/propane | [163] |
| propylene/propane | FT/Liquid | zirconia/AgNO3 | Ag+ | SF 20 propylene/propane | [164] |
| propylene/propane | FT/Liquid | TEG/AgBF4 | Ag+ | SF 60 propylene/propane | [165] |
| propylene/propane | FT/Liquid | AgBF4 | Ag+ | S 4.5 propylene | [166] |
| propylene/propane | FT/Liquid | PVDF/AgNO3 | Ag+ | SF 474 propylene/propane | [167] |
| propylene/propane | FT/Liquid | BMIM+BF4−/Ag | Ag+ | SF 17 propylene/propane | [168] |
| propylene/propane | FT/Liquid | Ag-BMImBF4 | Ag+ | NS | [169] |
| propylene/propane | FT/Liquid | AgNO3/PVDF (Millipore) | Ag+ | NS | [170] |
| propylene/propane | FT/Liquid | BMIM+BF4− | Cu | SF 5.2 propylene/propane | [93] |
| propylene/propane | FT/Liquid | PVDF/AgNO3 | Ag+ | SF 480 propylene/propane | [171] |
| propylene/propane | FT/Liquid | PVDF/AgNO3 | Ag+ | SF 490 propylene/propane | [172] |
| propylene/propane | FT/Liquid | [Ag(propene)x][Tf2N] | Ag+ | SF 3 propane/propene | [173] |
| propylene/propane | FT/Liquid | RTILs | Ag+ | SF 100 propylene/propane | [174] |
| propylene/propane | FT/Liquid | PVDF/AgNO3 | Ag+ | SF 270 propylene/propane | [175] |
| propylene/propane | FT/Liquid | BMImBF4 | Ag+ | SF 20 propylene/propane | [176] |
| propylene/propane | FT/Liquid | MOIM+NO3− | IL | SF 2.8 propylene/propane | [99] |
| propylene/propane | FT/Liquid | BMIM+BF4− | IL | SF 2.3 propylene/propane | [99] |
| propylene/propane | FT/Liquid | AgNO3 in hollow fiber membrane | Ag+ | 75% propylene removal | [177] |
| propylene/propane | FT/Liquid | (Emim,Ag)[BF4]−PICPM+PF6− | Ag+ | SF 7 propylene/propane | [178] |
| propylene/propane | FT/Liquid | (Emim,Ag)[Tf2N]−PICPM+Tf2N− | Ag+ | SF 7 propylene/propane | [178] |
| propylene/propane | FT/Liquid | (Emim,Ag)[Tf2N]-12HSA | Ag+ | SF 7 propylene/propane | [178] |
| propylene/propane | FT/Liquid | MOIM+BF4−/Cu | Cu | SF 2 propylene/propane | [179] |
| propylene/propane | FT/Liquid | PVDF/AgNO3 | Ag+ | SF 473.86 propylene/propane | [118] |
| propylene/propane | FT/Liquid | NMP | Ag+ | S 4.5 propylene | [180] |
| propylene/propane | FT/Polymer | PVA/AgSbF6 | Ag+ | S 125 propylene | [181] |
| propylene/propane | FT/Polymer | PVDFHFP/BMImBF4/AgBF4 | Ag+ | NS | [182] |
| propylene/propane | FT/Polymer | PE-g-AA-Ag+ | Cu | SF 21 propylene/propane | [60] |
| propylene/propane | FT/Polymer | PPO | Ag+ | SF 5.33 propylene/propane | [183] |
| propylene/propane | FT/Polymer | Cu/PVP | Cu | SF 10 propylene/propane | [184] |
| propylene/propane | FT/Polymer | AgNO3/PEG/Psf | Ag+ | SF 250 propylene/propane | [185] |
| propylene/propane | FT/Polymer | AgBF4-PVP | Ag+ | SF 140 propylene/propane | [124] |
| propylene/propane | FT/Polymer | POZ | Ag+ | SF 280 proylene/propane | [186] |
| propylene/propane | FT/Polymer | PEO | Ag+ | NS | [187] |
| propylene/propane | FT/Polymer | AgBF4-PVP | Ag+ | SF 140 propylene/propane | [188] |
| propylene/propane | FT/Polymer | AgBF4-POZ | Ag+ | SF 130 propylene/propane | [188] |
| propylene/propane | FT/Polymer | PVP/AgBF4 | Ag+ | NS | [189] |
| propylene/propane | FT/Polymer | PVP/AgBF4 | Ag+ | SF 60 propylene/propane | [190] |
| propylene/propane | FT/Polymer | PVP/AgNO3/Ppy | Ag+ | NS | [191] |
| propylene/propane | FT/Polymer | POZ | Ag+ | SF 5 propylene/propane | [192] |
| propylene/propane | FT/Polymer | PEP/AgBF4 | Ag+ | SF 55 propylene/propane | [94] |
| propylene/propane | FT/Polymer | PDMS/AgBF4 | Ag+ | SF 200 propylene/propane | [193] |
| propylene/propane | FT/Polymer | PHMV | Ag+ | S 336 propylene | [194] |
| propylene/propane | FT/Polymer | POZ | Ag+ | SF 65 propylene/propane | [195] |
| propylene/propane | FT/Polymer | PVP/silver salts | Ag+ | NS | [196] |
| propylene/propane | FT/Polymer | POZ/AgBF4 | Ag+ | SF 45 propylene/propane | [197] |
| propylene/propane | FT/Polymer | 6FDA–4MPD/DABA | Ag+ | S 10 propylene/propane | [198] |
| propylene/propane | FT/Polymer | BMIM+BF4 | Ag+ | SF 17 propylene/propane | [95] |
| propylene/propane | FT/Polymer | SBS/0.5Ag | Ag+ | S 80 propylene/propane | [199] |
| propylene/propane | FT/Polymer | Ag–sugar/BMIM+BF4− (0.05/1) | Ag+ | SF 12.9 propylene/propane | [200] |
| propylene/propane | FT/Polymer | PVC-g-P4VP | Ag+ | S 6 propylene | [201] |
| propylene/propane | FT/Polymer | PEI/Pebax2533/AgBF4 | Ag+ | SF 1000 propylene/propane | [202] |
| propylene/propane | FT/Polymer | PU/AgCF3SO3 (BASF ) | Ag+ | S 10 propylene | [203] |
| propylene/propane | FT/Polymer | PTFE (Mencor) | Ag+ | 60% propylene | [121] |
| propylene/propane | FT/Polymer | PP/AgBF4 | Ag+ | NS | [204] |
| propylene/propane | FT/Polymer | polymer membranes with inorganic nanoparticles uniformly dispersed | Zn | SF 18.08 propylene/propane | [205] |
| propylene/propane | FT/Polymer | Pebax® 1657/AgBF4 (Atofina) | Ag+ | SF 20.4 propylene/propane | [206] |
| propylene/propane | FT/Polymer | poly(vinylalcohol)/AgBF4/Al(NO3)3 | Ag+ | SF 17 propylene/propane | [98] |
| propylene/propane | FT/Polymer | (PVA)/AgBF4/Al(NO3)3 | Ag+ | NS | [98] |
| propylene/propane | FT/Polymer | PVP/AgBF4/Al(NO3)3/Ag2O | Ag+ | SF 21.7 propylene/propane | [100] |
| propylene/propane | FT/Polymer | CAF (CMS) | Ag+ | SF 50 propylene/propane | [207] |
| propylene/propane | FT/Polymer | SBS/Cu@MIL-101(Cr) MMM | Cu | S 2 propylene | [208] |
| propylene/propane | FT/Polymer | PE-g-AA-Ag+ | Ag+ | S 5 propane | [209] |
| propylene/propane | FT/Polymer | PE-g-AA-Cu+ | Cu+ | S 2.2 propane | [209] |
| propylene/propane | FT/Polymer | PE-g-AA-Cu2+ | Cu2+ | S 1.7 propane | [209] |
| propylene/propane | FT/Polymer | PEO-AgBF4 | Ag+ | NS | [151] |
| Separated Gases | Name of the Material | Selectivity or Sep Factor | Permeability or Permeance | Temp (K) | Pressure (bar) | Ref. |
|---|---|---|---|---|---|---|
| ethylene/ethane | Carbonized BPDA-pp’ODA Polyimide | SF 5 ethylene/ethane | P 1 ethylene (×10−8 mol m−2 s−1 Pa−1) | 373 | 1.013 | [81] |
| ethylene/ethane | Matrimid® 5218 (Huntsman) | S 12 ethane | P 14.4 (barrer) | 308 | NS | [215] |
| ethylene/ethane | Matrimid® 5218 (Huntsman) | S 12 ethylene/ethane | P 14–15 ethylene (barrer) | 308 | 3.447 | [216] |
| ethylene/ethane | Matrimid | SF 60 ethylene/ethane | P 4.8 × 10−7 ethylene; P 1.6 × 10−9 ethane (mol·Pa−1·m−2·s−1) | NS | NS | [217] |
| ethylene/ethane | 6FDA/BPDA-DAM | SF >20 | P 10 ethylene GPU | 308 | 20.265 | [218] |
| ethylene/ethane | PIM-6FDA-OH | SF 17.5 ethylene/ethane | P 10 ethylene (barrer) | 308 | 20.265 | [219] |
| ethylene/ethane | Matrimid and 6FDA/BPDA-DAM | NS | NS | 308 | 8.04 | [220] |
| ethylene/ethane | 6FDA/BPDA-DAM | S 3.9 ethylene/ethane | P 15.9 ethylene; P 4.0 ethane (GPU) | 298 | 5.15 | [221] |
| propylene/propane | 6FDA/BPDA–DDBT | S 22 propylene | P 26 GPU propylene | 373 | 1.013 | [84] |
| propylene/propane | NTDA-BAHFDS | S 42 propane | P 26 GPU propylene/propane | 308 | 1.013 | [86] |
| propylene/propane | AlPO-14 | NS | NS | NS | NS | [222] |
| propylene/propane | 6FDA/BPDA-DAM | S 20.5 propylene/propane | P 17.5 propylene; P 0.85 propane (GPU) | 298 | 5.15 | [221] |
| propylene/propane | CMS/g-Al2O3 | SF 36 propylene/propane | P 9 GPU propylene | 298 | 1.3–4 | [223] |
| propylene/propane | 6FDA | S 50–60 propylene | P 8 propylene/propane [×10−9 mol/(m2 s Pa)] | 393 | 6.89 | [224] |
| propylene/propane | CMS membranes synthesized on mesoporous g-alumina support | SF 31 propylene/propane | P 1.0 [× 10−8 mol m−2 s−1 Pa−1] | 298 | 3.1 | [63] |
| propylene/propane | BPDA-DDBT/DABA | SF 13 propylene/propane | P 50 GPU propane | 373 | 1.013 | [82] |
| Separated Gases | Name of the Material | Selectivity or Sep Factor | Permeability or Permeance | Temp (K) | Pressure (bar) | Ref. |
|---|---|---|---|---|---|---|
| ethylene/ethane | CuCl-modified tubular γ-Al2O3 membrane | SF 1.4 ethylene/ethane | NS | 333 | 2.026 | [226] |
| ethylene/ethane | CuCl/NaX | NS | NS | 358 | 2 | [227] |
| ethylene/ethane | Na-ETS-10 | S 5 ethylene | NS | 298 | 1.013 | [228] |
| ethylene/ethane | AgA and AgX | NS | NS | 303 | 1.013 | [229] |
| ethylene/ethane | ZIF-4 and ZIF-zni | NS | NS | 293 | NS | [230] |
| ethylene/ethane | ZIF-4 | SF 1.71 ethane/ethylene | NS | 293 | up to 12 | [231] |
| ethylene/ethane | Ag-X | S 15.9 ethylene | P 9.04 ¹ | 303 | NS | [232] |
| ethylene/ethane | 6FDA-DAM:DABA | SF 9 ethylene/ethane | P 90 ethylene (barrer) | 308 | 3.44 | [233] |
| ethylene/ethane | ZIF-7 | NS | NS | NS | 0 | [77] |
| ethylene/ethane | ZIF-8 | S 2.8 ethylene | P 1.5 ethylene ¹ | 298 | 1 | [78] |
| ethylene/ethane | Cu3BTC2 | SF 7.1 ethylene/ethane | P 17 ¹ | 423 | 5 | [234] |
| ethylene/ethane | Cu3BTC2 | SF 7.1 ethylene/ethane | P 17¹ | 423 | 5 | [234] |
| ethylene/ethane | IRMOF-8 | S 1.43 Ethane/Ethylene | NS | 318 | 8 | [235] |
| ethylene/ethane | MIL-101 | SF 16.5 ethylene/ethane | NS | 303 | 1 | [236] |
| ethylene/ethane | MIL-100 | 111 ethylene/ethane | NS | 298 | 0.01 | [225] |
| ethylene/ethane | M–MOF-74 | SF 10 ethylyne/ethane | NS | 318 | 1 | [237] |
| ethylene/ethane | Mg2(dhtp) | S 1.4 ethylene/ethane | NS | 293 | 0.015 | [238] |
| ethylene/ethane | Co2(dhtp) | S 1.7 ethylene/ethane | NS | 293 | 0.015 | [238] |
| ethylene/ethane | ZIF-8 | S 0.48 ethylene/ethane | NS | 293 | 0.015 | [238] |
| ethylene/ethane | Fe2(dobdc) | NS | NS | 318 | NS | [101] |
| ethylene/ethane | CuBTC | NS | NS | 303; 373 | 0.01–5 | [239] |
| ethylene/ethane | ZIF-71 | SF 1.84 propane/propylene | NS | 293 | 1 | [240] |
| propylene/propane | Mg2(dhtp) | S 1.7 propylene/propane | NS | 293 | 0.015 | [238] |
| propylene/propane | Co2(dhtp) | S 2.9 propylene/propane | NS | 293 | 0.015 | [238] |
| propylene/propane | ZIF-8 | S 0.7 propylene/propane | NS | 293 | 0.015 | [238] |
| propylene/propane | Fe2(dobdc) | NS | NS | 318 | NS | [101] |
| propylene/propane | CuBTC | NS | NS | 303; 373 | 0.01 - 5 | [239] |
| propylene/propane | ZIF-8 | NS | NS | NS | 1 | [241] |
| propylene/propane | Basolite® C300 (BASF) | NS | NS | 323–373 | 5 | [79] |
| propylene/propane | 6FDA-Durene/DABAco-polyimides ZIF-8 | SF 27.38 propylene/propane | NS | 308 | 10.13 | [242] |
| propylene/propane | NbOFFIVE-1-Ni (KAUST-7) | NS | NS | 298 | 1 | [243] |
| propylene/propane | ZIF-9 | SF 1.39 ethane/ethylene | NS | 293 | 1 | [240] |
| propylene/propane | Zr-fum-fcu-MOF | NS | NS | 328 | NS | [80] |
| propylene/propane | MIL-100 | 70 propylene/propane | NS | 298 | 0.01 | [225] |
| Separated Gases | Name of the Material | Carrier | Temp (K) | Pressure (bar) | Lifetime | Ref. |
|---|---|---|---|---|---|---|
| 1-butene/n-butane | ILMs in PVDF substrates | Ag+ | NS | 0.14 | at least 600 h | [256] |
| ethylene/ethane | Fluoropore FP-010/AgNO3 | Ag+ | 298 | 1.01 | at least 100 h | [90] |
| ethylene/ethane | EPDM-SPEEK | Ag+ | 298 | 3 | at least 1680 h | [131] |
| ethylene/ethane | ZnCl2/[BMIM][Cl] | Cu | 298 | 1.1 | 150 h | [257] |
| ethylene/ethane | AgBF4/PEO | Ag+ | 296 | 1.72 | at least 16 h | [139] |
| ethylene/ethane | AgNO3/polyethersulfone | Ag+ | 298 | 0.09 | 1440 h | [141] |
| ethylene/ethane | PA 12-PTMO/AgBF4 | Ag+ | 295 | 3.44 | 72 h | [142] |
| ethylene/ethane | EPDM | Ag+ | 298 | 3 | over 3360 h | [136] |
| ethylene/ethane | AgNO3/polyethersulfone | Ag+ | 298 | 2 | 504 h | [144] |
| ethylene/ethane | SiO2 Poly(sodium acrylate) Ag+ | Ag+ | 373 | 2 | at least 5 h | [147] |
| ethylene/ethane | Pebax® 2533/AgBF4 | Ag+ | 296 | 3.44 | 7 days | [115] |
| ethylene/ethane | Psf/AgNO3 | Ag+ | NS | 1 | 1440 h | [148] |
| ethylene/ethane | PEO-AgBF4 | Ag+ | 296 | 7.9 | at least 20 h | [151] |
| i-butene/i-butane | (PTMSP-g-AA-Ag+) | Ag+ | 298 | NS | at least 1008 h | [258] |
| isoprene/n-pentane | SPEEK-AgNO3 | Ag+ | 333 | 101.325 | 100 h | [259] |
| pentene/pentane | Select | Ag+ | 298 | 1.013 | 48 h | [260] |
| propylene/propane | POZ/AgNO3/SiO2 | Ag+ | 293 | 2.75 | 160 h | [154] |
| propylene/propane | PVP/Nano Au | Au | 298 | 1.013 | 2 days | [96] |
| propylene/propane | POZ | Ag+ | 293 | 2.75 | 14 days | [156] |
| propylene/propane | PVDF-HFP/BMImBF4–Ag+ | Ag+ | 293–323 | 0.5–3 | 10 days | [157] |
| propylene/propane | AgNO3/Al2O3 | Ag+ | 298 | 1 | at least 4320 h | [158] |
| propylene/propane | TiO2-PEO-AgBF4 | Ag+ | 298 | 1 | less than 196 h | [160] |
| propylene/propane | Permylene | Ag+ | 298 | 5.56 | over 1000 h | [161] |
| propylene/propane | POZ/P154AgNO3/BMIM+NO3− | Ag+ | NS | NS | 150 h | [163] |
| propylene/propane | TEG/AgBF4 | Ag+ | 293–298 | 1.013 | 1440–2160 h | [165] |
| propylene/propane | PVDF/AgNO3 | Ag+ | 298 | 1.2 | 2880 h | [167] |
| propylene/propane | BMIM+BF4−/Ag | Ag+ | NS | 2.75 | at least 100 h | [168] |
| propylene/propane | AgNO3/PVDF | Ag+ | 298 | 1.2 | 3–4 weeks | [170] |
| propylene/propane | PVDF/AgNO3 | Ag+ | 298 | 1.2 | 3–4 weeks | [175] |
| propylene/propane | PVDF/AgNO3 | Ag+ | NS | NS | 2880 h | [118] |
| propylene/propane | NMP | Ag+ | 293 | 1.2–2.2 | 60 h | [180] |
| propylene/propane | Cu/PVP | Cu | 298 | 1.38 | 168 h | [184] |
| propylene/propane | AgBF4-PVP | Ag+ | NS | NS | at least 100 h | [124] |
| propylene/propane | POZ | Ag+ | 296 | 1.38 | 50 h | [186] |
| propylene/propane | AgBF4-PVP | Ag+ | NS | NS | at least 100 h | [188] |
| propylene/propane | AgBF4-POZ | Ag+ | NS | NS | at least 100 h | [188] |
| propylene/propane | PVP/AgBF4 | Ag+ | NS | 2.76 | 720 h | [190] |
| propylene/propane | PEP/AgBF4 | Ag+ | 293 | 2.758 | 150 h | [94] |
| propylene/propane | PDMS/AgBF4 | Ag+ | NS | 1.38 | at least 5.8 h | [193] |
| propylene/propane | PTFE | Ag+ | 298 | 1.2 | 2 months | [121] |
| propylene/propane | poly(vinylalcohol)/AgBF4/Al(NO3)3 | Ag+ | NS | 3 | 145 h | [98] |
| propylene/propane | CAF (CMS) | Ag+ | 298 | 5.15 | over 9 months | [207] |
| propylene/propane | PEO-AgBF4 | Ag+ | 296 | 7.9 | at least 20 h | [151] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, D.M.V.d.; Dutra, L.d.S.; Way, D.; Amaral, N.; Wegenast, F.; Scaldaferri, M.C.; Jesus, N.; Pinto, J.C. A Bibliometric Survey of Paraffin/Olefin Separation Using Membranes. Membranes 2019, 9, 157. https://doi.org/10.3390/membranes9120157
Miranda DMVd, Dutra LdS, Way D, Amaral N, Wegenast F, Scaldaferri MC, Jesus N, Pinto JC. A Bibliometric Survey of Paraffin/Olefin Separation Using Membranes. Membranes. 2019; 9(12):157. https://doi.org/10.3390/membranes9120157
Chicago/Turabian StyleMiranda, Débora Micheline Vaz de, Luciana da Silva Dutra, Débora Way, Nicolis Amaral, Frederico Wegenast, Maria Clara Scaldaferri, Normando Jesus, and José Carlos Pinto. 2019. "A Bibliometric Survey of Paraffin/Olefin Separation Using Membranes" Membranes 9, no. 12: 157. https://doi.org/10.3390/membranes9120157
APA StyleMiranda, D. M. V. d., Dutra, L. d. S., Way, D., Amaral, N., Wegenast, F., Scaldaferri, M. C., Jesus, N., & Pinto, J. C. (2019). A Bibliometric Survey of Paraffin/Olefin Separation Using Membranes. Membranes, 9(12), 157. https://doi.org/10.3390/membranes9120157