Unique Metabolomic Profile of Skeletal Muscle in Chronic Limb Threatening Ischemia
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Populations
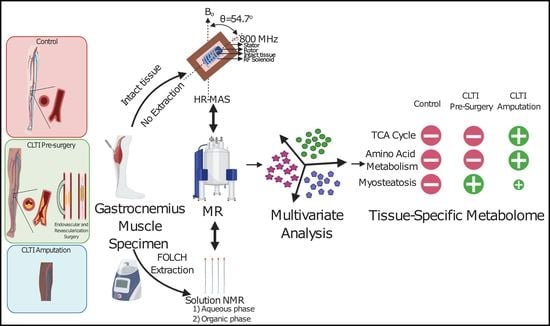
2.2. Muscle Specimen Collection
2.3. Chemicals
2.4. Metabolite Extraction
2.5. Sample Preparation and NMR Acquisition
HR-MAS NMR on Intact Gastrocnemius Muscle Specimens
2.6. Data Processing and Analysis
2.7. Metabolites Assignment
2.8. Statistical Analysis
3. Results
3.1. Patient Physical and Clinical Demographics
3.2. Metabolomic Analysis of Gastrocnemius Muscle
3.3. CLTI Display Biomarkers of Ischemic Metabolism at Amputation but Not Prior to Surgery
3.4. Dysregulated Amino Acid Metabolisms Are Distinguising Charactertistics of CLTI
3.5. Lipidomic Differences in CLTI Muscle Are Indicative of Myosteatosis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, A.D.; Hawkins, A.T.; Schaumeier, M.J.; de Vos, M.S.; Conte, M.S.; Nguyen, L.L. Predictors of major amputation despite patent bypass grafts. J. Vasc. Surg. 2016, 63, 1279–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, M.S.; Bandyk, D.F.; Clowes, A.W.; Moneta, G.L.; Seely, L.; Lorenz, T.J.; Namini, H.; Hamdan, A.D.; Roddy, S.P.; Belkin, M.; et al. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 2006, 43, 742–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodney, P.P.; Likosky, D.S.; Cronenwett, J.L. Predicting ambulation status one year after lower extremity bypass. J. Vasc. Surg. 2009, 49, 1431–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.M.; Kalbaugh, C.A.; Blackhurst, D.W.; Cass, A.L.; Trent, E.A.; Langan, E.M., 3rd; Youkey, J.R. Determinants of functional outcome after revascularization for critical limb ischemia: An analysis of 1000 consecutive vascular interventions. J. Vasc. Surg. 2006, 44, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.W.; Montgomery, P.S.; Parker, D.E. Physical activity is a predictor of all-cause mortality in patients with intermittent claudication. J. Vasc. Surg. 2008, 47, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Liu, K.; Tian, L.; Criqui, M.H.; Guralnik, J.M.; Ferrucci, L.; Liao, Y.; McDermott, M.M. Leg strength predicts mortality in men but not in women with peripheral arterial disease. J. Vasc. Surg. 2010, 52, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, M.M.; Liu, K.; Ferrucci, L.; Tian, L.; Guralnik, J.M.; Liao, Y.; Criqui, M.H. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J. Am. Coll. Cardiol. 2011, 57, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Liu, K.; Ferrucci, L.; Criqui, M.H.; Tian, L.; Guralnik, J.M.; Tao, H.; McDermott, M.M. The Walking Impairment Questionnaire stair-climbing score predicts mortality in men and women with peripheral arterial disease. J. Vasc. Surg. 2012, 55, 1662–1673. [Google Scholar] [CrossRef] [Green Version]
- McDermott, M.M.; Liu, K.; Tian, L.; Guralnik, J.M.; Criqui, M.H.; Liao, Y.; Ferrucci, L. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: A longitudinal study. J. Am. Coll. Cardiol. 2012, 59, 1159–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Liu, K.; Ferrucci, L.; Criqui, M.H.; Tian, L.; Guralnik, J.M.; Tao, H.; McDermott, M.M. Declining walking impairment questionnaire scores are associated with subsequent increased mortality in peripheral artery disease. J. Am. Coll. Cardiol. 2013, 61, 1820–1829. [Google Scholar] [CrossRef] [Green Version]
- Leeper, N.J.; Myers, J.; Zhou, M.; Nead, K.T.; Syed, A.; Kojima, Y.; Caceres, R.D.; Cooke, J.P. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J. Vasc. Surg. 2013, 57, 728–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissanen, T.T.; Vajanto, I.; Hiltunen, M.O.; Rutanen, J.; Kettunen, M.I.; Niemi, M.; Leppanen, P.; Turunen, M.P.; Markkanen, J.E.; Arve, K.; et al. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am. J. Pathol. 2002, 160, 1393–1403. [Google Scholar] [CrossRef] [Green Version]
- Pipinos, I.I.; Judge, A.R.; Selsby, J.T.; Zhu, Z.; Swanson, S.A.; Nella, A.A.; Dodd, S.L. The myopathy of peripheral arterial occlusive disease: Part. 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc. Endovascular. Surg. 2008, 42, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Yamaguchi, D.J.; Schmidt, C.A.; Zeczycki, T.N.; Shaikh, S.R.; Brophy, P.; Green, T.D.; Tarpey, M.D.; Karnekar, R.; Goldberg, E.J.; et al. Extensive skeletal muscle cell mitochondriopathy distinguishes critical limb ischemia patients from claudicants. JCI Insight 2018, 3, e123235. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P.; Hiatt, W.R. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc. Med. 2000, 5, 55–59. [Google Scholar] [CrossRef]
- Bhat, H.K.; Hiatt, W.R.; Hoppel, C.L.; Brass, E.P. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation 1999, 99, 807–812. [Google Scholar] [PubMed]
- Brass, E.P.; Hiatt, W.R.; Green, S. Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: A point-counterpoint discussion. Vasc. Med. 2004, 9, 293–301. [Google Scholar] [CrossRef]
- Pipinos, I.I.; Shepard, A.D.; Anagnostopoulos, P.V.; Katsamouris, A.; Boska, M.D. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J. Vasc. Surg. 2000, 31, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Pipinos, I.I.; Judge, A.R.; Zhu, Z.; Selsby, J.T.; Swanson, S.A.; Johanning, J.M.; Baxter, B.T.; Lynch, T.G.; Dodd, S.L. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic. Biol. Med. 2006, 41, 262–269. [Google Scholar] [CrossRef]
- Pipinos, I.I.; Swanson, S.A.; Zhu, Z.; Nella, A.A.; Weiss, D.J.; Gutti, T.L.; McComb, R.D.; Baxter, B.T.; Lynch, T.G.; Casale, G.P. Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R290–R296. [Google Scholar] [CrossRef]
- Schocke, M.; Esterhammer, R.; Greiner, A. High-energy phosphate metabolism in the exercising muscle of patients with peripheral arterial disease. Vasa 2008, 37, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wurdeman, S.R.; Myers, S.A.; Johanning, J.M.; Pipinos, I.I.; Stergiou, N. External work is deficient in both limbs of patients with unilateral PAD. Med. Eng. Phys. 2012, 34, 1421–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, D.J.; Casale, G.P.; Koutakis, P.; Nella, A.A.; Swanson, S.A.; Zhu, Z.; Miserlis, D.; Johanning, J.M.; Pipinos, I.I. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J. Transl. Med. 2013, 11, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutakis, P.; Weiss, D.J.; Miserlis, D.; Shostrom, V.K.; Papoutsi, E.; Ha, D.M.; Carpenter, L.A.; McComb, R.D.; Casale, G.P.; Pipinos, I.I. Oxidative damage in the gastrocnemius of patients with peripheral artery disease is myofiber type selective. Redox Biol. 2014, 2, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Keller, U.; Oberhansli, R.; Huber, P.; Widmer, L.K.; Aue, W.P.; Hassink, R.I.; Muller, S.; Seelig, J. Phosphocreatine content and intracellular pH of calf muscle measured by phosphorus NMR spectroscopy in occlusive arterial disease of the legs. Eur. J. Clin. Investig. 1985, 15, 382–388. [Google Scholar] [CrossRef]
- Hands, L.J.; Bore, P.J.; Galloway, G.; Morris, P.J.; Radda, G.K. Muscle Metabolism in Patients with Peripheral Vascular-Disease Investigated by P-31 Nuclear-Magnetic-Resonance Spectroscopy. Clin. Sci. 1986, 71, 283–290. [Google Scholar] [CrossRef]
- Zatina, M.A.; Berkowitz, H.D.; Gross, G.M.; Maris, J.M.; Chance, B. P-31 Nuclear-Magnetic-Resonance Spectroscopy-Noninvasive Biochemical-Analysis of the Ischemic Extremity. J. Vasc. Surg. 1986, 3, 411–420. [Google Scholar]
- Isbell, D.C.; Berr, S.S.; Toledano, A.Y.; Epstein, F.H.; Meyer, C.H.; Rogers, W.J.; Harthun, N.L.; Hagspiel, K.D.; Weltman, A.; Kramer, C.M. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J. Am. Coll. Cardiol. 2006, 47, 2289–2295. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.R.; Layec, G.; Trinity, J.D.; Kwon, O.S.; Zhao, J.; Reese, V.R.; Gifford, J.R.; Richardson, R.S. Increased skeletal muscle mitochondrial free radical production in peripheral arterial disease despite preserved mitochondrial respiratory capacity. Exp. Physiol. 2018, 103, 838–850. [Google Scholar] [CrossRef]
- McDermott, M.M.; Peterson, C.A.; Sufit, R.; Ferrucci, L.; Guralnik, J.M.; Kibbe, M.R.; Polonsky, T.S.; Tian, L.; Criqui, M.H.; Zhao, L.; et al. Peripheral artery disease, calf skeletal muscle mitochondrial DNA copy number, and functional performance. Vasc. Med. 2018, 23, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Freire, M.; Moore, A.Z.; Peterson, C.A.; Kosmac, K.; McDermott, M.M.; Sufit, R.L.; Guralnik, J.M.; Polonsky, T.; Tian, L.; Kibbe, M.R.; et al. Associations of Peripheral Artery Disease With Calf Skeletal Muscle Mitochondrial DNA Heteroplasmy. J. Am. Heart Assoc. 2020, 9, e015197. [Google Scholar] [CrossRef] [PubMed]
- Groennebaek, T.; Billeskov, T.B.; Schytz, C.T.; Jespersen, N.R.; Botker, H.E.; Olsen, R.K.J.; Eldrup, N.; Nielsen, J.; Farup, J.; De Paoli, F.V.; et al. Mitochondrial Structure and Function in the Metabolic Myopathy Accompanying Patients with Critical Limb Ischemia. Cells 2020, 9, 570. [Google Scholar]
- Gratl, A.; Frese, J.; Speichinger, F.; Pesta, D.; Frech, A.; Omran, S.; Greiner, A. Regeneration of Mitochondrial Function in Gastrocnemius Muscle in Peripheral Arterial Disease After Successful Revascularisation. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.E.; Schmidt, C.A.; Alleman, R.J.; Tsang, A.M.; Green, T.D.; Neufer, P.D.; Brown, D.A.; McClung, J.M. Mitochondrial therapy improves limb perfusion and myopathy following hindlimb ischemia. J. Mol. Cell Cardiol. 2016, 97, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.E.; Schmidt, C.A.; Green, T.D.; Spangenburg, E.E.; Neufer, P.D.; McClung, J.M. Targeted Expression of Catalase to Mitochondria Protects Against Ischemic Myopathy in High.-Fat Diet.-Fed Mice. Diabetes 2016, 65, 2553–2568. [Google Scholar] [CrossRef] [Green Version]
- McClung, J.M.; McCord, T.J.; Ryan, T.E.; Schmidt, C.A.; Green, T.D.; Southerland, K.W.; Reinardy, J.L.; Mueller, S.B.; Venkatraman, T.N.; Lascola, C.D.; et al. BAG3 (Bcl-2-Associated Athanogene-3) Coding Variant in Mice Determines Susceptibility to Ischemic Limb Muscle Myopathy by Directing Autophagy. Circulation 2017, 136, 281–296. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Giusti, B.; Takis, P.G.; Valente, S.; Carrabba, N.; Balzi, D.; Barchielli, A.; Marchionni, N.; Gensini, G.F.; et al. NMR-based metabolomics identifies patients at high risk of death within two years after acute myocardial infarction in the AMI-Florence II cohort. BMC Med. 2019, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- DeFilippis, A.P.; Trainor, P.J.; Hill, B.G.; Amraotkar, A.R.; Rai, S.N.; Hirsch, G.A.; Rouchka, E.C.; Bhatnagar, A. Identification of a plasma metabolomic signature of thrombotic myocardial infarction that is distinct from non-thrombotic myocardial infarction and stable coronary artery disease. PLoS ONE 2017, 12, e0175591. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Nie, X.; Xu, S.; Li, Y.; Huang, T.; Tang, H.; Wang, Y. Integrative metabonomics as potential method for diagnosis of thyroid malignancy. Sci. Rep. 2015, 5, 14869. [Google Scholar] [CrossRef] [Green Version]
- Khattri, R.B.; Sirusi, A.A.; Suh, E.H.; Kovacs, Z.; Merritt, M.E. The influence of Ho3+ doping on 13C DNP in the presence of BDPA. Phys. Chem. Chem. Phys. 2019, 21, 18629–18635. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High.-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. 2019, 58, 968–994. [Google Scholar] [CrossRef] [PubMed]
- Shet, K.; Siddiqui, S.M.; Yoshihara, H.; Kurhanewicz, J.; Ries, M.; Li, X. High.-resolution magic angle spinning NMR spectroscopy of human osteoarthritic cartilage. NMR Biomed. 2012, 25, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opstad, K.S.; Bell, B.A.; Griffiths, J.R.; Howe, F.A. An investigation of human brain tumour lipids by high-resolution magic angle spinning 1H MRS and histological analysis. NMR Biomed. 2008, 21, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Tzika, A.A.; Astrakas, L.; Cao, H.; Mintzopoulos, D.; Andronesi, O.C.; Mindrinos, M.; Zhang, J.; Rahme, L.G.; Blekas, K.D.; Likas, A.C.; et al. Combination of high-resolution magic angle spinning proton magnetic resonance spectroscopy and microscale genomics to type brain tumor biopsies. Int. J. Mol. Med. 2007, 20, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.E.; Brophy, P.; Lin, C.T.; Hickner, R.C.; Neufer, P.D. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: A comparison with in situ measurements. J. Physiol. 2014, 592, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ravanbakhsh, S.; Liu, P.; Bjorndahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C.; et al. Correction: Accurate, Fully-Automated NMR Spectral Profiling for Metabolomics. PLoS ONE 2015, 10, e0132873. [Google Scholar] [CrossRef]
- Osis, G.; Webster, K.; Harris, A.; Lee, H.; Chen, C.; Fang, L.; Romero, M.; Khattri, R.; Merritt, M.; Verlander, J.; et al. Regulation of renal NaDC1 expression and citrate excretion by NBCe1-A. Am. J. Physiol. Ren. Physiol. 2019, 317, F489–F501. [Google Scholar] [CrossRef]
- Lohr, K.E.; Khattri, R.B.; Guingab-Cagmat, J.; Camp, E.F.; Merritt, M.E.; Garrett, T.J.; Patterson, J.T. Metabolomic profiles differ among unique genotypes of a threatened Caribbean coral. Sci. Rep. 2019, 9, 6067. [Google Scholar] [CrossRef]
- Myer, C.; Abdelrahman, L.; Banerjee, S.; Khattri, R.B.; Merritt, M.E.; Junk, A.K.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor metabolite profile of pseudoexfoliation glaucoma is distinctive. Mol. Omics. 2020, 16, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Downes, D.P.; Collins, J.H.P.; Lama, B.; Zeng, H.; Nguyen, T.; Keller, G.; Febo, M.; Long, J.R. Characterization of Brain Metabolism by Nuclear Magnetic Resonance. Chemphyschem 2019, 20, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2008, 36, D402–D408. [Google Scholar] [CrossRef] [Green Version]
- Krssak, M.; Lindeboom, L.; Schrauwen-Hinderling, V.; Szczepaniak, L.; Derave, W.; Lundbom, J.; Befroy, D.; Schick, F.; Machann, J.; Kreis, R.; et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2020, e4266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, H.; Chang, D.; Guo, F.; Pan, H.; Yang, Y. Metabolomics approach by H-1 NMR spectroscopy of serum reveals progression axes for asymptomatic hyperuricemia and gout. Arthritis Res. Ther. 2018, 20, 111. [Google Scholar] [CrossRef] [Green Version]
- Barba, I.; de Leon, G.; Martin, E.; Cuevas, A.; Aguade, S.; Candell-Riera, J.; Barrabes, J.; Garcia-Dorado, D. Nuclear magnetic resonance-based metabolomics predicts exercise-induced ischemia in patients with suspected coronary artery disease. Magn. Reson. Med. 2008, 60, 27–32. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, H.J.; Kwon, Y.K.; Ryu, D.H.; Kwon, T.H.; Hwang, G.S. 1H NMR-based metabolite profiling of plasma in a rat model of chronic kidney disease. PLoS ONE 2014, 9, e85445. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Y.T.; Miller, J.H.; Day, M.M.; Munger, J.C.; Brookes, P.S. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell Rep. 2018, 23, 2617–2628. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.R.; Swanson, S.A.; Haynatzki, G.; Koutakis, P.; Johanning, J.M.; Reppert, P.R.; Papoutsi, E.; Miserlis, D.; Zhu, Z.; Casale, G.P.; et al. Protein Concentration and Mitochondrial Content in the Gastrocnemius Predicts Mortality Rates in Patients With Peripheral Arterial Disease. Ann. Surg. 2014, 261, 605–610. [Google Scholar] [CrossRef]
- Matsubara, Y.; Matsumoto, T.; Aoyagi, Y.; Tanaka, S.; Okadome, J.; Morisaki, K.; Shirabe, K.; Maehara, Y. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J. Vasc. Surg. 2015, 61, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, S.H.; McDermott, M.M.; Sufit, R.L.; Kosmac, K.; Bugg, A.W.; Gonzalez-Freire, M.; Ferrucci, L.; Tian, L.; Zhao, L.; Gao, Y.; et al. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: An observational study. J. Transl. Med. 2016, 14, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.P.; Olson, K.C.; Gao, C.; Prosdocimo, D.A.; Zhou, M.Y.; Wang, Z.H.; Jeyaraj, D.; Youn, J.Y.; Ren, S.X.; Liu, Y.X.; et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 2016, 133, 2038–2049. [Google Scholar] [CrossRef]
- Uddin, G.M.; Zhang, L.Y.; Shah, S.; Fukushima, A.; Wagg, C.S.; Gopal, K.; Al Batran, R.; Pherwani, S.; Ho, K.L.; Boisvenue, J.; et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc. Diabetol. 2019, 18, 86. [Google Scholar] [CrossRef]
- Torres, M.J.; Kew, K.A.; Ryan, T.E.; Pennington, E.R.; Lin, C.T.; Buddo, K.A.; Fix, A.M.; Smith, C.A.; Gilliam, L.A.; Karvinen, S.; et al. 17beta-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle. Cell Metab. 2018, 27, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.J.; Ryan, T.E.; Lin, C.T.; Zeczycki, T.N.; Neufer, P.D. Impact of 17beta-estradiol on complex I kinetics and H2O2 production in liver and skeletal muscle mitochondria. J. Biol. Chem. 2018, 293, 16889–16898. [Google Scholar] [CrossRef] [Green Version]
- Solsona-Vilarrasa, E.; Fucho, R.; Torres, S.; Nunez, S.; Nuno-Lambarri, N.; Enrich, C.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Cholesterol enrichment in liver mitochondria impairs oxidative phosphorylation and disrupts the assembly of respiratory supercomplexes. Redox Biol. 2019, 24, 101214. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Beavers, D.P.; Houston, D.K.; Harris, T.B.; Hue, T.F.; Koster, A.; Newman, A.B.; Simonsick, E.M.; Studenski, S.A.; Nicklas, B.J.; et al. Associations between body composition and gait-speed decline: Results from the Health, Aging, and Body Composition study. Am. J. Clin. Nutr. 2013, 97, 552–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive Adipose Tissue Infiltration in Skeletal Muscle in Individuals With Obesity, Diabetes Mellitus, and Peripheral Neuropathy: Association With Performance and Function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Critical Limb-Threatening Ischemia (CLTI) | ||||
|---|---|---|---|---|
| Characteristic | Control (n = 10) | Pre-Surgery (n = 10) | Amputation (n = 10) | p-Value (X2 or ANOVA) |
| Mean age (SD)—yr | 73.9 (7.8) | 64.5 (9.4) | 69.5 (6.2) | 0.043 A |
| Female sex—n (%) | 4 (40) | 0 (0) | 1 (10) | 0.044 |
| Overweight/Obese (BMI ≥ 25)—n (%) | 9 (90) | 7 (70) | 8 (80) | 0.535 |
| Ankle-brachial index (ABI)—(SD) | 1.1 (0.1) | 0.7 (0.3) | 0.5 (0.3) * | 0.014 A |
| Rutherford Classification—n (%) | ||||
| 0 | 10 (100) | 0 (0) | 0 (0) | <0.001 |
| 3 | 0 (0) | 4 (40) | 0 (0) | 0.093 |
| 4 | 0 (0) | 2 (20) | 4 (40) | 0.624 |
| 5 | 0 (0) | 4 (40) | 4 (40) | 0.646 |
| 6 | 0 (0) | 0 (0) | 2(20) | 0.454 |
| Medical history—n (%) | ||||
| Diabetes mellitus type I or II | 4 (40) | 6 (60) | 9 (90) | 0.065 |
| Hypertension | 7 (70) | 10 (100) | 10 (100) | 0.536 |
| Hyperlipidemia | 4 (40) | 10 (100) | 10 (100) | 0.006 |
| Coronary artery disease | 1 (10) | 6 (60) | 9 (90) | 0.001 |
| Renal disease | 0 (0) | 1 (10) | 3 (30) | 0.133 |
| Chronic obstructive pulmonary disease | 1 (10) | 4 (40) | 3 (30) | 0.303 |
| Tobacco use—n (%) | 4 (40) | 7 (70) | 9 (90) | 0.058 |
| Former smoker | 3 (30) | 4 (40) | 7 (70) | 0.175 |
| Current smoker | 1 (10) | 3 (30) | 2 (20) | 0.535 |
| Medication used—n (%) | ||||
| Aspirin | 4 (40) | 8 (80) | 9 (90) | 0.035 |
| Statin | 4 (40) | 10 (100) | 10 (100) | <0.001 |
| Angiotensin-converting enzyme (ACE) inhibitor | 5 (50) | 5 (50) | 6 (60) | 0.874 |
| Cilostazol | 0 (0) | 3 (30) | 4 (40) | 0.089 |
| Previous vascular intervention—n (%) | 0 (0) | 0 (0) | 5 (50) | 0.003 |
| Spectra Range (ppm) | Metabolite | Peak Pattern | VIP Scores | |
|---|---|---|---|---|
| Solution NMR (Aqueous Phase) | HR-MAS | |||
| 1.31–1.33 | Lactate | d | ~1.9 | ~1.5 |
| 3.55 | Glycine | s | ~1.8 | N.A. |
| 2.39–2.40 | Succinate | s | ~1.7 | N.A. |
| 8.32–8.35 | Inosine | s | ~1.6 | N.A. |
| 4.04–4.06 | O-Phosphoethanolamine | t | ~1.6 | N.A. |
| 3.03–3.04 | Creatinine + PCr | s | ~1.5 | N.A. |
| 1.46–1.48 | Alanine | d | ~1.2 | N.A. |
| 2.32–2.36 | Glutamate | m | ~1.2 | N.A. |
| 3.70–3.71 | 3-methyl histidine | s | ~1.2 | N.A. |
| 3.10 | Malonate | s | ~1.1 | N.A. |
| 6.88–6.90 | Tyrosine | d | ~1.1 | N.A. |
| 7.40–7.44 | Phenylalanine | t | ~1.1 | N.A. |
| 2.37–2.38 | Pyruvate | s | ~1.1 | N.A. |
| 2.22–2.25 | 2-Aminoadipate | s | ~1.1 | N.A. |
| 0.84–0.95 | CH3-lipids | m | N.A. | ~1.4 |
| 5.19–5.26 | CH-glycerol | m | N.A. | ~1.4 |
| 1.14–1.43 | (CH2)n lipids | m | N.A. | ~1.4 |
| 4.25–4.34 | CH2OCOR (glyceryl) | dd | N.A. | ~1.4 |
| 1.52–1.64 | (CH2–CH2–CO–) lipids | s | N.A. | ~1.3 |
| 1.93–2.11 | (CH=CH–CH2–CH2) lipids | m | N.A. | ~1.3 |
| 5.26–5.39 | –CH= lipids | m | N.A. | ~1.3 |
| 0.94–0.96 | Leucine | t | N.A. | ~1.1 |
| 2.70–2.90 | HC=CH–CH2–HC=CH | m | N.A. | ~1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattri, R.B.; Kim, K.; Thome, T.; Salyers, Z.R.; O’Malley, K.A.; Berceli, S.A.; Scali, S.T.; Ryan, T.E. Unique Metabolomic Profile of Skeletal Muscle in Chronic Limb Threatening Ischemia. J. Clin. Med. 2021, 10, 548. https://doi.org/10.3390/jcm10030548
Khattri RB, Kim K, Thome T, Salyers ZR, O’Malley KA, Berceli SA, Scali ST, Ryan TE. Unique Metabolomic Profile of Skeletal Muscle in Chronic Limb Threatening Ischemia. Journal of Clinical Medicine. 2021; 10(3):548. https://doi.org/10.3390/jcm10030548
Chicago/Turabian StyleKhattri, Ram B., Kyoungrae Kim, Trace Thome, Zachary R. Salyers, Kerri A. O’Malley, Scott A. Berceli, Salvatore T. Scali, and Terence E. Ryan. 2021. "Unique Metabolomic Profile of Skeletal Muscle in Chronic Limb Threatening Ischemia" Journal of Clinical Medicine 10, no. 3: 548. https://doi.org/10.3390/jcm10030548
APA StyleKhattri, R. B., Kim, K., Thome, T., Salyers, Z. R., O’Malley, K. A., Berceli, S. A., Scali, S. T., & Ryan, T. E. (2021). Unique Metabolomic Profile of Skeletal Muscle in Chronic Limb Threatening Ischemia. Journal of Clinical Medicine, 10(3), 548. https://doi.org/10.3390/jcm10030548