Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study
Abstract
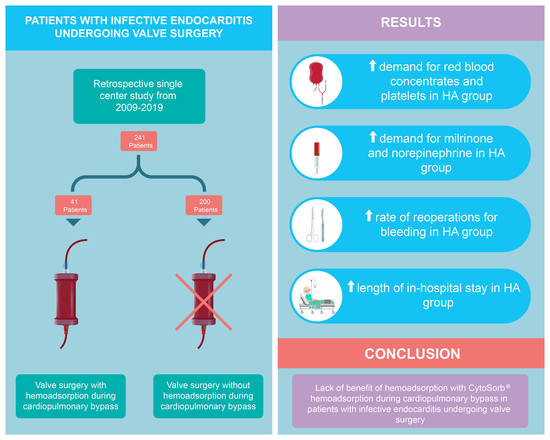
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Perioperative Data
3.3. Intensive Care Unit Data
3.4. Postoperative Results
3.5. Laboratory Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalani, T.; Chu, V.H.; Park, L.P.; Cecchi, E.; Corey, G.R.; Durante-Mangoni, E.; Fowler, V.G., Jr.; Gordon, D.; Grossi, P.; Hannan, M.; et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013, 173, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Langley, S.M.; Stafford, H.; Lowes, J.A.; Livesey, S.A.; Monro, J.L. Surgery for active culture-positive endocarditis: Determinants of early and late outcome. Ann. Thorac. Surg. 2000, 69, 1448–1454. [Google Scholar] [CrossRef]
- Cabell, C.H.; Abrutyn, E.; Fowler, V.G., Jr.; Hoen, B.; Miro, J.M.; Corey, G.R.; Olaison, L.; Pappas, P.; Anstrom, K.J.; Stafford, J.A.; et al. Use of surgery in patients with native valve infective endocarditis: Results from the International Collaboration on Endocarditis Merged Database. Am. Heart J. 2005, 150, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, N.S.; Finney, S.J.; Gordon, S.E.; Quinlan, G.J.; Evans, T.W. Modified criteria for the systemic inflammatory response syndrome improves their utility following cardiac surgery. Chest 2014, 145, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C. Biocompatibility in cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 1997, 11, 376–382. [Google Scholar] [CrossRef]
- Crawford, T.C.; Magruder, J.T.; Grimm, J.C.; Suarez-Pierre, A.; Sciortino, C.M.; Mandal, K.; Zehr, K.J.; Conte, J.V.; Higgins, R.S.; Cameron, D.E.; et al. Complications After Cardiac Operations: All Are Not Created Equal. Ann. Thorac. Surg. 2017, 103, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, J.; Arevalo, A.; Tamayo, E.; Sarria, C.; Aguilar-Blanco, E.M.; Heredia, M.; Almansa, R.; Rico, L.; Iglesias, V.; Bermejo-Martin, J.F. Cytokine profiles linked to fatal outcome in infective prosthetic valve endocarditis. APMIS 2014, 122, 526–529. [Google Scholar] [CrossRef]
- Araujo, I.R.; Ferrari, T.C.; Teixeira-Carvalho, A.; Campi-Azevedo, A.C.; Rodrigues, L.V.; Guimaraes Junior, M.H.; Barros, T.L.; Gelape, C.L.; Sousa, G.R.; Nunes, M.C. Cytokine Signature in Infective Endocarditis. PLoS ONE 2015, 10, e0133631. [Google Scholar] [CrossRef]
- Biancari, F.; Lahtinen, J.; Lepojarvi, S.; Rainio, P.; Salmela, E.; Pokela, R.; Lepojarvi, M.; Satta, J.; Juvonen, T.S. Preoperative C-reactive protein and outcome after coronary artery bypass surgery. Ann. Thorac. Surg. 2003, 76, 2007–2012. [Google Scholar] [CrossRef]
- Hennein, H.A.; Ebba, H.; Rodriguez, J.L.; Merrick, S.H.; Keith, F.M.; Bronstein, M.H.; Leung, J.M.; Mangano, D.T.; Greenfield, L.J.; Rankin, J.S. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J. Thorac. Cardiovasc. Surg. 1994, 108, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Mihaljevic, T.; Tonz, M.; von Segesser, L.K.; Pasic, M.; Grob, P.; Fehr, J.; Seifert, B.; Turina, M. The influence of leukocyte filtration during cardiopulmonary bypass on postoperative lung function. A clinical study. J. Thorac. Cardiovasc. Surg. 1995, 109, 1138–1145. [Google Scholar] [CrossRef] [Green Version]
- Coraim, F.I.; Wolner, E. Continuous hemofiltration for the failing heart. New Horiz. 1995, 3, 725–731. [Google Scholar] [PubMed]
- Scholz, M.; Simon, A.; Matheis, G.; Dzemali, O.; Henrich, D.; Kleine, P.; Wimmer-Reinecker, G.; Moritz, A. Leukocyte filtration fails to limit functional neutrophil activity during cardiac surgery. Inflamm. Res. 2002, 51, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Trager, K.; Skrabal, C.; Fischer, G.; Datzmann, T.; Schroeder, J.; Fritzler, D.; Hartmann, J.; Liebold, A.; Reinelt, H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass—A case series. Int. J. Artif. Organs 2017, 40, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidari, Z.; Wendt, D.; Thielmann, M.; Mackowiak, M.; Neuhauser, M.; Jakob, H.; Ruhparwar, A.; El-Gabry, M. Intraoperative Hemoadsorption in Patients with Native Mitral Valve Infective Endocarditis. Ann. Thorac. Surg. 2020, 110, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Poli, E.C.; Alberio, L.; Bauer-Doerries, A.; Marcucci, C.; Roumy, A.; Kirsch, M.; De Stefano, E.; Liaudet, L.; Schneider, A.G. Cytokine clearance with CytoSorb(R) during cardiac surgery: A pilot randomized controlled trial. Crit. Care 2019, 23, 108. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.H.; Rinoesl, H.; Dragosits, K.; Ristl, R.; Hoffelner, F.; Opfermann, P.; Lamm, C.; Preissing, F.; Wiedemann, D.; Hiesmayr, M.J.; et al. Effect of hemoadsorption during cardiopulmonary bypass surgery—A blinded, randomized, controlled pilot study using a novel adsorbent. Crit. Care 2016, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Winchester, J.; Albright, R.L.; Capponi, V.J.; Choquette, M.D.; Kellum, J.A. Cytokine removal with a novel adsorbent polymer. Blood Purif. 2004, 22, 428–434. [Google Scholar] [CrossRef]
- Schadler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jorres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhart, K.; Meier-Hellmann, A.; Beale, R.; Forst, H.; Boehm, D.; Willatts, S.; Rothe, K.F.; Adolph, M.; Hoffmann, J.E.; Boehme, M.; et al. Open randomized phase II trial of an extracorporeal endotoxin adsorber in suspected Gram-negative sepsis. Crit. Care Med. 2004, 32, 1662–1668. [Google Scholar] [CrossRef]
- Hassan, K.; Kannmacher, J.; Wohlmuth, P.; Budde, U.; Schmoeckel, M.; Geidel, S. Cytosorb Adsorption During Emergency Cardiac Operations in Patients at High Risk of Bleeding. Ann. Thorac. Surg. 2019, 108, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Angheloiu, G.O.; Gugiu, G.B.; Ruse, C.; Pandey, R.; Dasari, R.R.; Whatling, C. Ticagrelor Removal from Human Blood. JACC Basic Transl. Sci. 2017, 2, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.Y.; Sin, Y.K.; Lim, C.H.; Tan, T.E.; Lim, S.L.; Chao, V.T.; Chua, Y.L. Surgical management of infective endocarditis: An analysis of early and late outcomes. Eur. J. Cardiothorac. Surg. 2015, 47, 826–832. [Google Scholar] [CrossRef]
- Gaca, J.G.; Sheng, S.; Daneshmand, M.A.; O’Brien, S.; Rankin, J.S.; Brennan, J.M.; Hughes, G.C.; Glower, D.D.; Gammie, J.S.; Smith, P.K. Outcomes for endocarditis surgery in North America: A simplified risk scoring system. J. Thorac. Cardiovasc. Surg. 2011, 141, 98–106.e2. [Google Scholar] [CrossRef] [Green Version]
- Kellum, J.A.; Venkataraman, R.; Powner, D.; Elder, M.; Hergenroeder, G.; Carter, M. Feasibility study of cytokine removal by hemoadsorption in brain-dead humans. Crit. Care Med. 2008, 36, 268–272. [Google Scholar] [CrossRef]
- Hadoke, P.W.; Macdonald, L.; Logie, J.J.; Small, G.R.; Dover, A.R.; Walker, B.R. Intra-vascular glucocorticoid metabolism as a modulator of vascular structure and function. Cell. Mol. Life Sci. 2006, 63, 565–578. [Google Scholar] [CrossRef]
- Lambden, S.; Creagh-Brown, B.C.; Hunt, J.; Summers, C.; Forni, L.G. Definitions and pathophysiology of vasoplegic shock. Crit. Care 2018, 22, 174. [Google Scholar] [CrossRef] [Green Version]
- Tschaikowsky, K.; Sagner, S.; Lehnert, N.; Kaul, M.; Ritter, J. Endothelin in septic patients: Effects on cardiovascular and renal function and its relationship to proinflammatory cytokines. Crit. Care Med. 2000, 28, 1854–1860. [Google Scholar] [CrossRef]
- Weil, M.H.; Henning, R.J.; Puri, V.K. Colloid oncotic pressure: Clinical significance. Crit. Care Med. 1979, 7, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Verheij, J.; van Lingen, A.; Beishuizen, A.; Christiaans, H.M.; de Jong, J.R.; Girbes, A.R.; Wisselink, W.; Rauwerda, J.A.; Huybregts, M.A.; Groeneveld, A.B. Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensiv. Care Med. 2006, 32, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Prigent, H.; Maxime, V.; Annane, D. Clinical review: Corticotherapy in sepsis. Crit. Care 2004, 8, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellers, M.M.; Stallone, J.N. Sympathy for the devil: The role of thromboxane in the regulation of vascular tone and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1978–H1986. [Google Scholar] [CrossRef] [Green Version]
- Hosgood, S.A.; Moore, T.; Kleverlaan, T.; Adams, T.; Nicholson, M.L. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J. Transl. Med. 2017, 15, 216. [Google Scholar] [CrossRef]
- Hawchar, F.; Laszlo, I.; Oveges, N.; Trasy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Javanbakht, M.; Trevor, M.; Rezaei, H.M.; Rahimi, K.; Branagan-Harris, M.; Degener, F.; Adam, D.; Preissing, F.; Scheier, J.; Cook, S.F.; et al. Ticagrelor Removal by CytoSorb((R)) in Patients Requiring Emergent or Urgent Cardiac Surgery: A UK-Based Cost-Utility Analysis. Pharmacoecon. Open 2020, 4, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Trager, K.; Fritzler, D.; Fischer, G.; Schroder, J.; Skrabal, C.; Liebold, A.; Reinelt, H. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: A case series. Int. J. Artif. Organs 2016, 39, 141–146. [Google Scholar] [CrossRef]
- Nemeth, E.; Kovacs, E.; Racz, K.; Soltesz, A.; Szigeti, S.; Kiss, N.; Csikos, G.; Koritsanszky, K.B.; Berzsenyi, V.; Trembickij, G.; et al. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin. Transpl. 2018, 32, e13211. [Google Scholar] [CrossRef] [Green Version]
- Diab, M.; Platzer, S.; Guenther, A.; Sponholz, C.; Scherag, A.; Lehmann, T.; Velichkov, I.; Hagel, S.; Bauer, M.; Brunkhorst, F.M.; et al. Assessing efficacy of CytoSorb haemoadsorber for prevention of organ dysfunction in cardiac surgery patients with infective endocarditis: REMOVE-protocol for randomised controlled trial. BMJ Open 2020, 10, e031912. [Google Scholar] [CrossRef] [Green Version]


| Patient Characteristics | HA (n = 41) | Control (n = 200) | Stddiff | p |
|---|---|---|---|---|
| Age, years | 66.1 ± 23.7 | 65.4 ± 14.9 | 0.036 | 0.854 |
| Female | 3 (8.0%) | 44 (21.9%) | 0.397 | 0.039 |
| BMI | 27.6 ± 11.5 | 25.9 ± 5.4 | 0.193 | 0.345 |
| Ejection fraction, % | 55.6 ± 13.3 | 56.3 ± 10.2 | −0.059 | 0.752 |
| Diabetes | 14 (34.5%) | 41 (20.6%) | −0.315 | 0.302 |
| Current Smoker | 8 (19.2%) | 47 (23.3%) | 0.101 | 0.625 |
| Platelet aggregation inhibitor | 19 (46.5%) | 115 (57.6%) | 0.222 | 0.389 |
| Peripheral artery disease | 3 (6.4%) | 16 (8.0%) | 0.062 | 0.705 |
| Preoperative stroke | 9 (21.8%) | 62 (30.9%) | 0.206 | 0.302 |
| Renal disease | 13 (32.5%) | 29 (14.4%) | −0.437 | 0.146 |
| Dialysis | 2 (5.7%) | 8 (4.2%) | −0.070 | 0.660 |
| COPD | 9 (23%) | 16 (7.8%) | −0.430 | 0.194 |
| Hypertension | 20 (48.5%) | 104 (52.1%) | 0.072 | 0.793 |
| Hypercholesteremia | 17 (40.4%) | 64 (31.9%) | −0.179 | 0.543 |
| NYHA III or IV | 20 (48.8%) | 87 (43.6%) | −0.104 | 0.702 |
| Preoperative AF | 2 (4.3%) | 25 (12.6%) | 0.300 | 0.196 |
| Prior MI | 3 (6.1%) | 12 (5.8%) | −0.013 | 0.937 |
| Emergency | 5 (12.9%) | 23 (11.7%) | −0.036 | 0.850 |
| EuroSCORE II, % | 7.8 (4.8 to 12.5) | 8.6 (7.2 to 10.3) | 0.219 | 0.185 |
| CRP, mg/L | 1 (0.1 to 14.4) | 0.2 (0.1 to 0.4) | 0.003 | 0.076 |
| Fibrinogen, g/L | 3.1 (2.8 to 3.4) | 2.6 (2.2 to 3.0) | 0.508 | 0.184 |
| Hemoglobin, g/L | 72.4 (54.1 to 96.9) | 59.3 (43.0 to 81.6) | 0.211 | 0.637 |
| WBC, counts/nL | 11.7 (8.3 to 16.5) | 9.8 (7.4 to 12.8) | 0.242 | 0.816 |
| Platelets, counts/nL | 142 (98 to 207) | 107 (76 to 151) | 0.190 | 0.949 |
| Perioperative Details | HA (n = 41) | Control (n = 200) | Stddiff | p |
|---|---|---|---|---|
| Perfusion time, min | 110 (80 to 150) | 138 (130 to 146) | 0.365 | 0.327 |
| Aortic clamping time, min | 92.7 ± 83.1 | 106.4 ± 48.3 | −0.202 | 0.308 |
| IV inotropes before surgery | 6 (14.2%) | 39 (19.6%) | 0.145 | 0.487 |
| Aortic valve | 33 (81.1%) | 143 (71.3%) | −0.230 | 0.258 |
| Mitral valve | 14 (35.3%) | 95 (47.5%) | 0.251 | 0.306 |
| Tricuspid valve | 1 (2.8%) | 11 (5.6%) | 0.137 | 0.393 |
| Severe insufficiency | 9 (22.8%) | 61 (30.3%) | 0.170 | 0.480 |
| Procedure Groups | 0.469 | |||
| ● CABG & Valve(s) | 2 (3.7%) | 19 (9.6%) | −0.238 | |
| ● CABG &Valve(s) & Other | 1 (3.5%) | 14 (7.2%) | −0.168 | |
| ● Valve(s) & Other | 16 (38.3%) | 75 (37.5%) | 0.015 | |
| ● Valve(s) only | 22 (54.6%) | 91 (45.7%) | 0.179 | |
| Assist Device | 0.141 | |||
| ● IABP | 1 (3.0%) | 10 (4.9%) | −0.098 | |
| ● ECMO | 2 (4.1%) | 1 (0.4%) | 0.247 |
| Intensive Care Unit Data | HA (n = 41) | Control (n = 200) | Stddiff | p |
|---|---|---|---|---|
| Administration of | ||||
| ● Epinephrine | 23 (57.1%) | 89 (44.7%) | −0.250 | 0.365 * |
| ● Dobutamine | 1 (3.5%) | 7 (3.6%) | 0.006 | 0.612 * |
| ● Milrinone | 17 (42.2%) | 34 (17.2%) | −0.567 | 0.046 * |
| ● Nitroglycerine | 2 (4.2%) | 29 (14.4%) | 0.357 | 0.057 |
| ● Norepinephrine | 36 (88.4%) | 106 (52.8%) | −0.848 | 0.001 * |
| ● RBC | 27 (65.2%) | 61 (30.6%) | −0.738 | 0.003 |
| ● Tranexamic acid | 1 (1.9%) | 8 (3.9%) | 0.122 | 0.497 |
| ● Haemate | 3 (7.6%) | 7 (3.3%) | −0.190 | 0.241 |
| ● FFP | 24 (58.3%) | 49 (24.6%) | −0.730 | 0.075 * |
| ● Fibrinogen | 13 (31.2%) | 29 (14.6%) | −0.405 | 0.194 |
| ● PCC | 4 (8.6%) | 25 (12.7%) | 0.132 | 0.489 |
| ● Platelets | 15 (36.7%) | 20 (9.8%) | −0.673 | 0.013 |
| Intubation >72 h | 9 (20.8%) | 20 (9.9%) | −0.305 | 0.406 |
| Drainage >800 mL within 12 h | 18 (43.7%) | 48 (23.8%) | −0.430 | 0.128 |
| Length of ICU stay, days | 5.1 (3.8 to 6.8) | 3.2 (2.7 to 3.8) | 0.546 | 0.230 |
| RRT | 3 (6.4%) | 13 (6.5%) | 0.007 | 0.970 |
| Reoperation for bleeding | 14 (34.0%) | 15 (7.7%) | −0.685 | 0.011 |
| Reoperation later than 24 h | 10 (23.6%) | 10 (4.8%) | −0.559 | 0.062 |
| Postoperative Details | HA (n = 41) | Control (n = 200) | Stddiff | p |
|---|---|---|---|---|
| AF at discharge (NOAF) | 17 (42.2%) | 50 (24.8%) | −0.374 | 0.129 |
| Delirium | 20 (47.9%) | 53 (26.7%) | −0.448 | 0.095 |
| In-hospital mortality | 3 (6.8%) | 20 (10.0%) | 0.118 | 0.485 |
| Length of hospital stay | 15.2 (11.8 to 19.6) | 9.0 (7.1 to 11.3) | 0.463 | 0.017 |
| MACCE | 5 (11.1%) | 27 (13.4%) | 0.069 | 0.704 |
| Neurological complication | 20 (49.7%) | 58 (29.0%) | −0.433 | 0.110 |
| Permanent pacemaker | 2 (5.6%) | 39 (19.6%) | 0.432 | 0.021 |
| Pulmonary infection | 4 (9.7%) | 17 (8.3%) | −0.049 | 0.782 |
| Postoperative renal failure | 5 (13.2%) | 40 (20.0%) | 0.185 | 0.360 |
| Postoperative sepsis | 6 (14.4%) | 12 (6.2%) | −0.273 | 0.108 |
| Postoperative stroke | 2 (4.3%) | 9 (4.3%) | −0.002 | 0.991 |
| Renal replacement therapy | 3 (6.4%) | 13 (6.5%) | 0.007 | 0.970 |
| Day 1 to 3 | HA | Time, Days | Interaction | |||
|---|---|---|---|---|---|---|
| Parameter | Coefficient (95% CI) | p | Coefficient (95% CI) | p | Coefficient (95% CI) | p |
| CRP (mg/L) | −4.82 (−47.7 to 38.1) | 0.826 | 41.8 (35.8 to 47.9) | <0.001 | 2.88 (−18.5 to 24.2) | 0.791 |
| Fibrinogen (g/L) | −0.03 (−0.43 to 0.37) | 0.890 | 0.49 (0.41 to 0.56) | <0.001 | 0.08 (−0.09 to 0.24) | 0.356 |
| Hemoglobin (g/L) | −4.33 (−9.62 to 0.95) | 0.108 | −2.79 (−3.68 to −1.91) | <0.001 | 1.36 (−0.87 to 3.59) | 0.231 |
| WBC (counts/nL) | −3.94 (−6.33 to −1.55) | 0.001 | −0.63 (−1.05 to −0.21) | 0.003 | 1.78 (0.62 to 2.93) | 0.003 |
| Platelets (counts/nL) | −10.0 (−44.8 to 24.7) | 0.572 | 3.50 (−0.77 to 7.77) | 0.108 | 4.28 (−8.17 to 16.7) | 0.500 |
| Regulator | Function | Influence of HA |
|---|---|---|
| Cortisol | increases response to catecholamines via steroid receptors [33] | ↓ [27] |
| Thromboxane | vasoconstrictor and promoter of platelet aggregation [34] | ↓ [35] |
| Big-endothelin-1 | precursor of vasoconstricting Endothelin-1 [30] | ↓ [36] |
| Albumin | Maintenance of plasma colloid oncotic pressure [31] and effect on cardiac preload [32] | ↓ [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santer, D.; Miazza, J.; Koechlin, L.; Gahl, B.; Rrahmani, B.; Hollinger, A.; Eckstein, F.S.; Siegemund, M.; Reuthebuch, O.T. Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study. J. Clin. Med. 2021, 10, 564. https://doi.org/10.3390/jcm10040564
Santer D, Miazza J, Koechlin L, Gahl B, Rrahmani B, Hollinger A, Eckstein FS, Siegemund M, Reuthebuch OT. Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study. Journal of Clinical Medicine. 2021; 10(4):564. https://doi.org/10.3390/jcm10040564
Chicago/Turabian StyleSanter, David, Jules Miazza, Luca Koechlin, Brigitta Gahl, Bejtush Rrahmani, Alexa Hollinger, Friedrich S. Eckstein, Martin Siegemund, and Oliver T. Reuthebuch. 2021. "Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study" Journal of Clinical Medicine 10, no. 4: 564. https://doi.org/10.3390/jcm10040564
APA StyleSanter, D., Miazza, J., Koechlin, L., Gahl, B., Rrahmani, B., Hollinger, A., Eckstein, F. S., Siegemund, M., & Reuthebuch, O. T. (2021). Hemoadsorption during Cardiopulmonary Bypass in Patients with Endocarditis Undergoing Valve Surgery: A Retrospective Single-Center Study. Journal of Clinical Medicine, 10(4), 564. https://doi.org/10.3390/jcm10040564