The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature
Abstract
:1. Introduction
2. State-of-the-Art Review
2.1. Microbiota and Hypertension
2.2. The Role of SCFAs
2.3. Maternal Heritage and Genetic/Epigenetic Regulation
2.4. Inflammation and Immune System in Hypertension
2.5. The Role of Trimethylamine-N-Oxide
2.6. The Role of Lipopolysaccharides
2.7. The Role of Salt
2.8. Microbiota and Exercise
2.9. Nutrition and Stiffness
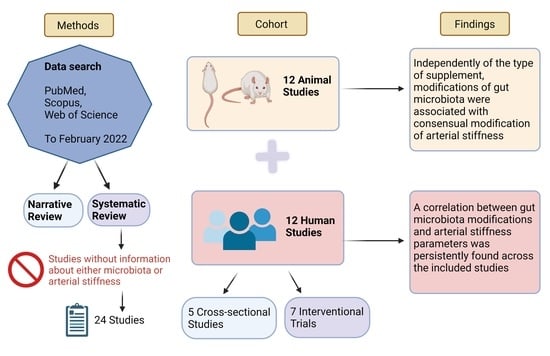
3. Systematic Review
3.1. Aim
3.2. Methods
3.2.1. Eligibility Criteria
3.2.2. Information Sources and Search
3.3. Results
3.4. Discussion
3.4.1. Animal Studies
| Authors | n | Marker of VA | Intervention | Duration | Effect on Vascular Ageing | Mechanisms Linked to Microbiota |
|---|---|---|---|---|---|---|
| Guirro, M., 2020 [77] | 48 | Neuraminidase circulating levels | Hesperidin treatment; two diets for 9 wk (n = 24): standard diet and cafeteria (CAF) diet | 9 weeks of diet + 8 weeks of hesperidin | CAF feeding resulted in increased endothelial dysfunction, arterial stiffness, and inflammation. Hesperidin supplementation reduced SBP and markers of arterial stiffness in CAF-fed rats | Urinary metabolites of hesperidin were positively correlated with Bacteroidaceae family. |
| Liu, H., 2020 [78] | 35 | PWV at the left common carotid artery | Gavage with feces from either healthy donors (controls) or myocardial infarction patients (CAD) + high fat diet | 12 weeks | Mice treated with CAD feces had higher vascular stiffness than controls (Controls: 2.75 ± 0.29 m/s vs. CAD: 3.59 ± 0.27 m/s; p = 0.043). No BP data. | In mice treated with CAD feces: increased LPS and pro-inflammatory cytokines; increased activated TH17 cells; reduced Treg cells. |
| Battson, M.L., 2019 [79] | 40 | AorticPWV (doppler) | Fecal transplantation, 10 controls and 10 obese mice received healthy microbiota, and 10 and 10 received obese microbiota | 8 weeks | Control mice receiving microbiota of obese subjects had higher PWV. Akkermansia abundance inversely related to PWV. No BP data. | Obese mice had reduced Clostridia and Oscillospira. Control mice and obese mice receiving microbiota of obese subjects had higher level of Bacteroides sp. Control mice receiving microbiota of obese subjects had reduced level of Akkermansia. |
| Natarajan, N., 2016 [29] | 10 | Aortic stiffness (PWV by doppler and ex vivo) | Gpr41 KO group vs Grp41 WT group | 3 and 6 months | At 6 months PWV was significantly higher in KO mice vs WT mice, with similar compliance in ex vivo analysis, suggesting functional vascular alteration. KO mice presented isolated systolic hypertension at baseline | Gpr41 (SCFA receptor) localizes in the vascular endothelium. Vascular endothelium is essential for SCFA-mediated vasodilation to occur, as vasodilation is absent in endothelium-denuded vessels ex vivo. |
| Edwards, J.M., 2020 [83] | 12 | Resistance arteries stiffness (ex vivo) | Ex vivo evaluation of vascular stiffness | Resistance arteries from male GF mice present increased vascular stiffness. No changes in vascular stiffness in arteries from female mice. No BP data. | Microbiota influenced the vasoconstriction response. | |
| Cross, T.W.L., 2017 [74] | 40 | Aortic PWV (doppler) | Ovariectomy vs sham surgery; soy-rich vs soy-free diet | 28 weeks | PWV was lowered with soy feeding but was not affected by ovariectomy. No BP data. | Soy-rich diet modified intestinal microbiota composition (lower F:B ratio). |
| Battson, M.L., 2018 [81] | 36 | Aortic PWV (doppler) | Standard diet (SD) (n 12) or Western diet (WD) (n 24) for 5 months, then WD mice were randomized to receive broad-spectrum antibiotic cocktail (WD + Abx) or placebo (n 12/group) for 2 months | 7 months | PWV progressively increased in WD mice during the 7-month intervention. In WD + Abx, PWV was completely normalized to SD levels. No BP data. | WD had increased Firmicutes and decreased Bacteroidetes and Actinobacteria. Abundance of numerous bacterial taxa were altered by diet; in particular, Bifidobacterium spp. were significantly more abundant in SD animals compared with WD. |
| Brunt, V.E., 2019 [82] | 73 | Aortic PWV (doppler); ex vivo intrinsic mechanical stiffness | Cocktail of broad-spectrum, poorly absorbed antibiotics in drinking water vs placebo. 4 groups: young controls (YC); young antibiotics (YA); old controls (OC); old antibiotics (OA). | 3–4 weeks | At baseline, PWV was higher in OC and OA vs YC (p < 0.01). PWV increased in YC but not in YA during intervention. In OA, PWV was reduced at the end of the intervention. Antibiotic treatment in old mice was associated with a partial improvement back towards young levels (p = 0.047 vs. OC). Aortic elastin protein expression was lower in OC vs. YC (p = 0.02), but was restored in OA. No BP modifications were registered. | Ageing was associated with greater alpha diversity. Old mice demonstrated several bacterial markers of gut dysbiosis and/or inflammation. Three-fold age-related increase in circulating plasma TMAO levels. In both young and old mice, antibiotic treatment suppressed TMAO levels. |
| Lee, D.M., 2018 [75] | 47 | Aortic PWV (doppler); ex vivo | (1) standard diet; (2) standard diet + dapagliflozin (60 mg dapagliflozin/kg diet). Controls (n = 11); Controls + dapa (n = 12); Diabetics (Db) (n = 12); Db + dapa (n = 12). | 8 weeks | Dapagliflozin treatment improved both endothelium-dependent dilatation (EDD) and Endothelium-independent dilation (EID) in Db mice. PWV was negatively and EID-EDD positively correlated with Akkermansia abundance. PWV was positively correlated with Firmicutes and F:B ratio. No BP data. | Significantly reduced richness and diversity in the Db + dapa group compared to controls. Bacteroidetes and Proteobacteria were influenced by dapagliflozin treatment in Db + dapa. Db + dapa had a significantly lower F:B ratio than the other treatment groups. Oscillospira was significantly reduced in the Db + dapa compared to all other groups |
| Lee, D.M., 2020 [76] | 48 | Aortic PWV (doppler) | Standard (SD) vs Western diet (WD). Indole-3-propionic acid (IPA) vs placebo. (1) SD + placebo, (2) WD +placebo, (3) SD + IPA, 4) WD + IPA. (n = 12 mice/group). | 5 months | IPA supplementation did not affect PWV in WD, but impaired PWV in SD. Bifidobacterium reduction by WD was related to PWV. No BP data. | WD feeding decreased Bifidobacterium. Reduced abundance of Bifidobacterium was observed in SD + IPA. |
| Trikha, S.R.J., 2021 [80] | 10 | Aortic PWV (doppler) | 2 age-matched male and 2 female (1 of each lean [LM], and 1 obese [OBM]) microbiota donors to form cohorts 1 and 2 of inoculated mice. | PWV was increased in OBM mice vs. GF mice. In cohort 2, OBM mice displayed a marked increase in PWV vs. LM mice. No BP data. | Mouse microbiota profiles clustered according to their transplant donor groups. Taxa appear to be driving this separation, Bacteroides ovatus and Parabacteroides diastonis were consistently associated with LM mice. |
3.4.2. Human Studies
3.5. Conclusions
| Authors | n | Marker of VA | Intervention | Duration | Effect on Vascular Ageing | Mechanisms Linked to Microbiota |
|---|---|---|---|---|---|---|
| Rodriguez-Mateos, A., 2018 [96] | 45 | cfPWV | DP1-10 group: cocoa extract with 690 mg (130 mg epicatechin; 560 mg DP2-10 procyanidins). DP2-10 group: cocoa extract with 560 mg (20 mg epicatechin; 540 mg DP2-10 procyanidins). Controls. | 1 month | DP1-10 group: decrease in PWV at 1 mo of −1.0 m/s (95% CI: −1.6, −0.4 m/s) compared with the control and of −0.8 m/s (95% CI: −1.4, −0.2 m/s) compared with DP2-10. Decrease in SBP at 1 month in both treatment groups. | Epicatechin is absorbed via the colon after catabolism by the microbiota; Pro-cyanidins are also subject to microbiome-mediated catabolism. |
| Istas, G., 2019 [89] | 66 | cfPWV; AIx | Aronia whole fruit capsule: 12 mg (poly)phenols; aronia extract capsule: 116 mg (poly)phenols. | Acute: 0–2 h Chronic: 0–12 weeks | No significant difference in PWV and BP. | The aronia extract group: higher abundance of Anaerostipes; the aronia whole fruit group: increases in Bacteroides. |
| Taniguchi, H., 2018 [92] | 33 | CAVI | Exercise program (n = 16) and control period (n = 17). | 10 weeks | Changes in Clostridium Difficile were positively correlated both with CAVI (r 0.31, p 0.02; no effect of exercise) and with SBP | Diversity and composition of microbiota were not affected by exercise; exercise increased the relative abundance of Oscillospira and decreased the abundance of C. Difficile |
| Menni, C., 2018 [85] | 617 | cfPWV | Observational study in female twins. | N/a | Carotid-femoral PWV is inversely correlated with gut microbiome diversity and with the abundance of specific microbes in the gut (Ruminococcaceae family bacteria). Analysis was adjusted for MAP. | N/a |
| Biruete, A., 2019 [86] | 10 | cfPWV | Observational study in hemodialysis patients. | N/a | Faecalibacterium spp. (with anti-inflammatory properties), was negatively associated with aortic PWV. F:B ratio was positively associated with SBP. | N/a |
| Ponziani, F.R., 2017 [95] | 39 | Carotid PWV | Patients with small intestinal bacterial overgrowth (SIBO). | N/a | PWV was increased in the SIBO group compared to the no-SIBO group (10.25 m/s vs 7.68 m/s; p = 0.002). dp-ucMGP levels (marker of low vitamin-K2 status) correlated with PWV in whole population. No BP data. | Dietary vitamin-K2 intake does not correlate with vitamin-K2 status (measured by dp-ucMGP serum levels). The gut microbiota is crucial for overcoming dietary vitamin-K2 insufficiencies. |
| Ried, K., 2018 [90] | 49 | cfPWV (tonometry) | Kyolic Aged Garlic Extract vs placebo. | 12 weeks | No significant differences in PWV between groups and intra-group before and after treatment. Garlic reduced SBP. | Increase of Lactobacillus and Clostridia species in the garlic group. Faecalibacterium prausnitzii markedly increased in the placebo group. |
| Hazim, S., 2016 [97] | 28 | cfPWV | Soy isoflavones acute supplementation. | 3 days | Acute soy intakes modified cfPWV only in equol producer subjects at 24 h; equol concentrations were significantly correlated with changes in cfPWV. No changes in BP. | N/a |
| Huang, J., 2020 [94] | 24 | AIx75; SEVR | Obese individuals underwent exercise: endurance/strength training 5 h/day, 6 days/week; diet: calorie-restricted. | 6 weeks | Significant increase of SEVR; reduction of AIx. No changes in BP. | Increase in intestinal microbial diversity; abundance of Lactobacillales, Bacilli, Streptococcaceae, and Veillonella were significantly reduced. Christensenellaceae were significantly enhanced; changes in Cronobacter, Lachnospiraceae UCG-003, and Helicobacter were all positively or negatively associated with the changes in SEVR, AIx75. |
| Dinakis, E., 2021 [87] | 69 | AASI | Observational study. | No associations were found between alpha diversity and AASI; no significant clustering patterns of AASI; Small but positive correlation between plasma butyrate levels and AASI. No BP data. | AASI was associated with lower abundance of Lactobacillus spp. and higher abundance of several species from the genus Clostridium. | |
| Liu, X., 2022 [91] | 12 | baPWV/FMD | 2 eggs/day in healthy young men. | 2 weeks | Egg consumption improved baPWV and FMD. No effect on inflammation and oxidative stress. No changes in BP. | No change in taxonomy, alpha and beta diversity; reduced tryptophan degradation. |
| Hsu, C.N., 2018 [88] | 86 | carotid-PWV (echo-tracking) | Observational study on children and adolescents with chronic kidney disease (CKD). | N/a | Carotid-PWV was elevated in children with CKD and eGFR category G2–G3 compared to those with eGFR category G1. 65% of children and adolescents with CKD G1–G3 had BP abnormalities on ABPM. | CKD children with an abnormal ABPM profile had lower abundance of the genus Prevotella; the abundances of genera Bifidobacterium and Lactobacillus were correlated with urinary TMAO level. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blacher, J.; Evans, A.; Arveiler, D.; Amouyel, P.; Ferrières, J.; Bingham, A.; Yarnell, J.; Haas, B.; Montaye, M.; Ruidavets, J.-B.; et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: The PRIME Study. J. Hum. Hypertens. 2010, 24, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, P.M. Early Vascular Aging in Hypertension. Front. Cardiovasc. Med. 2020, 7, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakatta, E.G.; Wang, M.; Najjar, S.S. Arterial Aging and Subclinical Arterial Disease are Fundamentally Intertwined at Macroscopic and Molecular Levels. Med. Clin. N. Am. 2009, 93, 583–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Hwang, S.-J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events the Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Lozupone, C.; Faust, K.; Raes, J.; Faith, J.J.; Frank, D.N.; Zaneveld, J.; Gordon, J.I.; Knight, R. Identifying genomic and metabolic features that can underlie early successional and opportunistic lifestyles of human gut symbionts. Genome Res. 2012, 22, 1974–1984. [Google Scholar] [CrossRef] [Green Version]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Levine, U.Y.; Looft, T.; Allen, H.K.; Stanton, T.B. Butyrate-producing bacteria, including mucin degraders, from the swine intestinal tract. Appl. Environ. Microbiol. 2013, 79, 3879–3881. [Google Scholar] [CrossRef] [Green Version]
- Nagano, Y.; Itoh, K.; Honda, K. The induction of Treg cells by gut-indigenous Clostridium. Curr. Opin. Immunol. 2012, 24, 392–397. [Google Scholar] [CrossRef]
- Ghaffari, S.; Abbasi, A.; Somi, M.H.; Moaddab, S.Y.; Nikniaz, L.; Kafil, H.S.; Ebrahimzadeh Leylabadlo, H. Akkermansia muciniphila: From its critical role in human health to strategies for promoting its abundance in human gut microbiome. Crit. Rev. Food Sci. Nutr. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Lin, J.; Isnard, S.; Fombuena, B.; Peng, X.; Marette, A.; Routy, B.; Messaoudene, M.; Chen, Y.; Routy, J.-P. The Bacterium Akkermansia muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation. Front. Immunol. 2020, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, K.; Greenland, P.; Stamler, J.; Liu, K.; Daviglus, M.L.; Nakagawa, H. Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men: The Chicago Western Electric Study. Am. J. Epidemiol. 2004, 159, 572–580. [Google Scholar] [CrossRef]
- Alonso, A.; de la Fuente, C.; Martín-Arnau, A.M.; de Irala, J.; Martínez, J.A.; Martínez-González, M.A. Fruit and vegetable consumption is inversely associated with blood pressure in a Mediterranean population with a high vegetable-fat intake: The Seguimiento Universidad de Navarra (SUN) Study. Br. J. Nutr. 2004, 92, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Tzoulaki, I.; Iliou, A.; Mikros, E.; Elliott, P. An Overview of Metabolic Phenotyping in Blood Pressure Research. Curr. Hypertens. Rep. 2018, 20, 78. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yu, B.; Alexander, D.; Mosley, T.H.; Heiss, G.; Nettleton, J.A.; Boerwinkle, E. Metabolomics and Incident Hypertension Among Blacks. Hypertension 2013, 62, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Loo, R.L.; Zou, X.; Appel, L.J.; Nicholson, J.K.; Holmes, E. Characterization of metabolic responses to healthy diets and association with blood pressure: Application to the Optimal Macronutrient Intake Trial for Heart Health (OmniHeart), a randomized controlled study. Am. J. Clin. Nutr. 2018, 107, 323–334. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, V.R.; Petrosino, J.F.; Bryan, R.M.J.; Durgan, D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugirdas, J.T.; Nawab, Z.M.; Klok, M. Acetate relaxation of isolated vascular smooth muscle. Kidney Int. 1987, 32, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutting, C.W.; Islam, S.; Daugirdas, J.T. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am. J. Physiol. Circ. Physiol. 1991, 261, H561–H567. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, F.V.; Nielsen, H.; Mulvany, M.J.; Hessov, I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 1990, 31, 1391–1394. [Google Scholar] [CrossRef] [Green Version]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiotaderived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [Green Version]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Tan, J.K.; McKenzie, C.; Mariño, E.; Macia, L.; Mackay, C.R. Metabolite-Sensing G Protein–Coupled Receptors—Facilitators of Diet-Related Immune Regulation. Annu. Rev. Immunol. 2017, 35, 371–402. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; MacIa, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Payen, C.; Guillot, A.; Paillat, L.; Fothi, A.; Dib, A.; Bourreau, J.; Schmitt, F.; Loufrani, L.; Aranyi, T.; Henrion, D.; et al. Pathophysiological adaptations of resistance arteries in rat offspring exposed in utero to maternal obesity is associated with sex-specific epigenetic alterations. Int. J. Obes. 2021, 45, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Itani, H.A.; McMaster, W.G.; Saleh, M.A.; Nazarewicz, R.R.; Mikolajczyk, T.P.; Kaszuba, A.M.; Konior, A.; Prejbisz, A.; Januszewicz, A.; Norlander, A.E.; et al. Activation of Human T Cells in Hypertension. Hypertension 2016, 68, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörffel, Y.; Lätsch, C.; Stuhlmüller, B.; Schreiber, S.; Scholze, S.; Burmester, G.R.; Scholze, J. Preactivated Peripheral Blood Monocytes in Patients With Essential Hypertension. Hypertension 1999, 34, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikolajczyk, T.P.; Guzik, T.J. Adaptive Immunity in Hypertension. Curr. Hypertens. Rep. 2019, 21, 68. [Google Scholar] [CrossRef] [Green Version]
- Norlander, A.E.; Madhur, M.S.; Harrison, D.G. Correction: The immunology of hypertension. J. Exp. Med. 2018, 215, 719. [Google Scholar] [CrossRef]
- Csiszar, A.; Ungvari, Z.; Edwards, J.G.; Kaminski, P.; Wolin, M.S.; Koller, A.; Kaley, G. Aging-Induced Phenotypic Changes and Oxidative Stress Impair Coronary Arteriolar Function. Circ. Res. 2002, 90, 1159–1166. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12065318 (accessed on 14 July 2013). [CrossRef] [Green Version]
- Wang, M.; Zhang, J.; Jiang, L.-Q.Q.; Spinetti, G.; Pintus, G.; Monticone, R.; Kolodgie, F.D.; Virmani, R.; Lakatta, E.G. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 2007, 50, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Boos, C.J.; Lip, G.Y.H. Elevated high-sensitive C-reactive protein, large arterial stiffness and atherosclerosis: A relationship between inflammation and hypertension? J. Hum. Hypertens. 2005, 19, 511–513. [Google Scholar] [CrossRef]
- Patel, R.S.; Al Mheid, I.; Morris, A.A.; Ahmed, Y.; Kavtaradze, N.; Ali, S.; Dabhadkar, K.; Brigham, K.; Hooper, W.C.; Alexander, R.W.; et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis 2011, 218, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Khera, R.; Corrales-Medina, V.F.; Townsend, R.R.; Chirinos, J.A. Inflammation and arterial stiffness in humans. Atherosclerosis 2014, 237, 381–390. [Google Scholar] [CrossRef]
- Wykretowicz, A.; Guzik, P.; Kąsinowski, R.; Krauze, T.; Bartkowiak, G.; Dziarmaga, M.; Wysocki, H. Augmentation index, pulse pressure amplification and superoxide anion production in patients with coronary artery disease. Int. J. Cardiol. 2005, 99, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Yasmin; McEniery, C.M.; Wallace, S.; Mackenzie, I.S.; Cockcroft, J.R.; Wilkinson, I.B. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 969–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schillaci, G.; Bartoloni, E.; Pucci, G.; Pirro, M.; Settimi, L.; Alunno, A.; Gerli, R.; Mannarino, E. Aortic stiffness is increased in polymyalgia rheumatica and improves after steroid treatment. Ann. Rheum. Dis. 2012, 71, 1151–1156. [Google Scholar] [CrossRef]
- Mäki-Petäjä, K.M.; Hall, F.C.; Booth, A.D.; Wallace, S.M.L.; Yasmin; Bearcroft, P.W.P.; Harish, S.; Furlong, A.; McEniery, C.M.; Brown, J.; et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006, 114, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef]
- Janeiro, M.; Ramírez, M.; Milagro, F.; Martínez, J.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Louca, P.; Menni, C.; Padmanabhan, S. Genomic Determinants of Hypertension with a Focus on Metabolomics and the Gut Microbiome. Am. J. Hypertens. 2020, 33, 473–481. [Google Scholar] [CrossRef]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, ehw582. [Google Scholar] [CrossRef]
- Brunt, V.E.; Casso, A.G.; Gioscia-Ryan, R.A.; Sapinsley, Z.J.; Ziemba, B.P.; Clayton, Z.S.; Bazzoni, A.E.; VanDongen, N.S.; Richey, J.J.; Hutton, D.A.; et al. Gut Microbiome-Derived Metabolite Trimethylamine N-Oxide Induces Aortic Stiffening and Increases Systolic Blood Pressure With Aging in Mice and Humans. Hypertension 2021, 78, 499–511. [Google Scholar] [CrossRef]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-Oxide: A Carnitine-Derived Metabolite That Prolongs the Hypertensive Effect of Angiotensin II in Rats. Can. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantero, M.A.; Guedes, M.R.A.; Fernandes, R.; Lollo, P.C.B. Trimethylamine N-oxide reduction is related to probiotic strain specificity: A systematic review. Nutr. Res. 2022, 104, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- Smiljanec, K.; Lennon, S.L. Sodium, hypertension, and the gut: Does the gut microbiota go salty? Am. J. Physiol.-Hear. Circ. Physiol. 2019, 317, H1173–H1182. [Google Scholar] [CrossRef] [PubMed]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yun, C.C. Mechanisms of the Regulation of the Intestinal Na+/H+ Exchanger NHE3. J. Biomed. Biotechnol. 2010, 2010, 238080. [Google Scholar] [CrossRef] [Green Version]
- Linz, D.; Wirth, K.; Linz, W.; Heuer, H.O.O.; Frick, W.; Hofmeister, A.; Heinelt, U.; Arndt, P.; Schwahn, U.; Böhm, M.; et al. Antihypertensive and Laxative Effects by Pharmacological Inhibition of Sodium-Proton-Exchanger Subtype 3–Mediated Sodium Absorption in the Gut. Hypertension 2012, 60, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Aihara, E.; Montrose, M.H.; Shull, G.E.; Hassett, D.J.; Worrell, R.T. Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. Am. J. Physiol. Liver Physiol. 2013, 305, G697–G711. [Google Scholar] [CrossRef] [Green Version]
- Laubitz, D.; Harrison, C.A.; Midura-Kiela, M.T.; Ramalingam, R.; Larmonier, C.B.; Chase, J.H.; Caporaso, J.G.; Besselsen, D.G.; Ghishan, F.K.; Kiela, P.R. Reduced Epithelial Na+/H+ Exchange Drives Gut Microbial Dysbiosis and Promotes Inflammatory Response in T Cell-Mediated Murine Colitis. PLoS ONE 2016, 11, e0152044. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Z.; Yu, K.; Ding, R.; Ye, K.; Dai, C.; Xu, X.; Zhou, G.; Li, C. High-Salt Diet Has a Certain Impact on Protein Digestion and Gut Microbiota: A Sequencing and Proteome Combined Study. Front. Microbiol. 2017, 8, 1838. [Google Scholar] [CrossRef] [Green Version]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Yi, B.; Titze, J.; Rykova, M.; Feuerecker, M.; Vassilieva, G.; Nichiporuk, I.; Schelling, G.; Morukov, B.; Choukèr, A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: A longitudinal study. Transl. Res. 2015, 166, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 Promotes Angiotensin II–Induced Hypertension and Vascular Dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [Green Version]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- De Bruyne, T.; Steenput, B.; Roth, L.; De Meyer, G.R.Y.; Dos Santos, C.N.; Valentová, K.; Dambrova, M.; Hermans, N. Dietary polyphenols targeting arterial stiffness: Interplay of contributing mechanisms and gut microbiome-related Metabolism. Nutrients 2019, 11, 578. [Google Scholar] [CrossRef] [Green Version]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Vlachopoulos, C.; Alexopoulos, N.; Stefanadis, C. Effects of nutrition on arterial rigidity and reflected waves. Sang Thromb. Vaiss. 2007, 19, 479–486. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809. [Google Scholar] [CrossRef] [Green Version]
- Richter, C.K.; Skulas-Ray, A.C.; Fleming, J.A.; Link, C.J.; Mukherjea, R.; Krul, E.S.; Kris-Etherton, P.M. Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: A dose-response randomised controlled trial. Br. J. Nutr. 2017, 117, 1403–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cross, T.W.L.; Zidon, T.M.; Welly, R.J.; Park, Y.M.; Britton, S.L.; Koch, L.G.; Rottinghaus, G.E.; De Godoy, M.R.C.; Padilla, J.; Swanson, K.S.; et al. Soy improves cardiometabolic health and cecal microbiota in female low-fit rats. Sci. Rep. 2017, 7, 9261. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018, 17, 62. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.M.; Ecton, K.E.; Trikha, S.R.J.; Wrigley, S.D.; Thomas, K.N.; Battson, M.L.; Wei, Y.; Johnson, S.A.; Weir, T.L.; Gentile, C.L. Microbial metabolite indole-3-propionic acid supplementation does not protect mice from the cardiometabolic consequences of a Western diet. Am. J. Physiol. - Gastrointest. Liver Physiol. 2020, 319, G51–G62. [Google Scholar] [CrossRef]
- Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Alcaide-Hidalgo, J.M.; Canela, N.; Arola, L.; Mayneris-Perxachs, J. Metabolomics elucidates dose-dependent molecular beneficial effects of hesperidin supplementation in rats fed an obesogenic diet. Antioxidants 2020, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Tian, R.; Wang, H.; Feng, S.; Li, H.; Xiao, Y.; Luan, X.; Zhang, Z.; Shi, N.; Niu, H.; et al. Gut microbiota from coronary artery disease patients contributes to vascular dysfunction in mice by regulating bile acid metabolism and immune activation. J. Transl. Med. 2020, 18, 382. [Google Scholar] [CrossRef]
- Battson, M.L.; Lee, D.M.; Li Puma, L.C.; Ecton, K.E.; Thomas, K.N.; Febvre, H.P.; Chicco, A.J.; Weir, T.L.; Gentile, C.L. Gut microbiota regulates cardiac ischemic tolerance and aortic stiffness in obesity. Am. J. Physiol. Circ. Physiol. 2019, 317, 1210–1220. [Google Scholar] [CrossRef]
- Trikha, S.R.J.; Lee, D.M.; Ecton, K.E.; Wrigley, S.D.; Vazquez, A.R.; Litwin, N.S.; Thomas, K.N.; Wei, Y.; Battson, M.L.; Johnson, S.A.; et al. Transplantation of an obesity-associated human gut microbiota to mice induces vascular dysfunction and glucose intolerance. Gut Microbes 2021, 13, 1940791. [Google Scholar] [CrossRef]
- Battson, M.L.; Lee, D.M.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E468–E477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Richey, J.J.; Zigler, M.C.; Cuevas, L.M.; Gonzalez, A.; Vázquez-Baeza, Y.; Battson, M.L.; Smithson, A.T.; Gilley, A.D.; et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 2019, 597, 2361–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, J.M.; Roy, S.; Tomcho, J.C.; Schreckenberger, Z.J.; Chakraborty, S.; Bearss, N.R.; Saha, P.; McCarthy, C.G.; Vijay-Kumar, M.; Joe, B.; et al. Microbiota are critical for vascular physiology: Germ-free status weakens contractility and induces sex-specific vascular remodeling in mice. Vascul. Pharmacol. 2020, 125–126, 106633. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.A.; Castorena-Gonzalez, J.A.; Ramirez-Perez, F.I.; Jia, G.; Hill, M.A.; Reyes-Aldasoro, C.C.; Sowers, J.R.; Martinez-Lemus, L.A. Arterial Stiffening in Western Diet-Fed Mice Is Associated with Increased Vascular Elastin, Transforming Growth Factor-β, and Plasma Neuraminidase. Front. Physiol. 2016, 7, 285. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.-F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef] [Green Version]
- Biruete, A.; Allen, J.M.; Kistler, B.M.; Jeong, J.H.; Fitschen, P.J.; Swanson, K.S.; Wilund, K.R. Gut Microbiota and Cardiometabolic Risk Factors in Hemodialysis Patients. Top. Clin. Nutr. 2019, 34, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dinakis, E.; Nakai, M.; Gill, P.A.; Yiallourou, S.; Sata, Y.; Muir, J.; Carrington, M.; Head, G.A.; Kaye, D.M.; Marques, F.Z. The Gut Microbiota and Their Metabolites in Human Arterial Stiffness. Heart Lung Circ. 2021, 30, 1716–1725. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Lu, P.-C.; Lo, M.-H.; Lin, I.-C.; Chang-Chien, G.-P.; Lin, S.; Tain, Y.-L. Gut Microbiota-Dependent Trimethylamine N-Oxide Pathway Associated with Cardiovascular Risk in Children with Early-Stage Chronic Kidney Disease. Int. J. Mol. Sci. 2018, 19, 3699. [Google Scholar] [CrossRef] [Green Version]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of aronia berry (poly)phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Sali, A. The Effect of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front. Nutr. 2018, 5, 122. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Sun, J.; Tu, J.; Wang, Z.; Tao, J.; Chen, J. Egg consumption improves vascular and gut microbiota function without increasing inflammatory, metabolic, and oxidative stress markers. FOOD Sci. Nutr. 2022, 10, 295–304. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef] [PubMed]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liao, J.; Fang, Y.; Deng, H.; Yin, H.; Shen, B.; Hu, M. Six-Week Exercise Training With Dietary Restriction Improves Central Hemodynamics Associated With Altered Gut Microbiota in Adolescents With Obesity. Front. Endocrinol. (Lausanne). 2020, 11, 569085. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Pompili, M.; Di Stasio, E.; Zocco, M.A.; Gasbarrini, A.; Flore, R. Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth via vitamin K2-dependent mechanisms. World J. Gastroenterol. 2017, 23, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Weber, T.; Skene, S.S.; Ottaviani, J.I.; Crozier, A.; Kelm, M.; Schroeter, H.; Heiss, C. Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: Randomized, controlled, double-masked intervention trial. Am. J. Clin. Nutr. 2018, 108, 1229–1237. [Google Scholar] [CrossRef]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Kappel, B.A.; De Angelis, L.; Heiser, M.; Ballanti, M.; Stoehr, R.; Goettsch, C.; Mavilio, M.; Artati, A.; Paoluzi, O.A.; Adamski, J.; et al. Cross-omics analysis revealed gut microbiome-related metabolic pathways underlying atherosclerosis development after antibiotics treatment. Mol. Metab. 2020, 36, 100976. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Li, X.; Cao, Y.; Chan, A.T.; Rimm, E.B.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Duration and Life-Stage of Antibiotic Use and Risks of All-Cause and Cause-Specific Mortality: Prospective Cohort Study. Circ. Res. 2020, 126, 364–373. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Nie, X.-Y.; Chen, X.-M.; Lin, X.-X.; Tang, K.; Zeng, W.-T.; Mei, W.-Y.; Liu, L.-J.; Long, M.; Yao, F.-J.; et al. The Role of Macrolide Antibiotics in Increasing Cardiovascular Risk. J. Am. Coll. Cardiol. 2015, 66, 2173–2184. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.Y.S.; Chan, E.W.; Anand, S.; Worsley, A.J.; Wong, I.C.K. Managing Cardiovascular Risk of Macrolides: Systematic Review and Meta-Analysis. Drug Saf. 2017, 40, 663–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorelik, E.; Masarwa, R.; Perlman, A.; Rotshild, V.; Abbasi, M.; Muszkat, M.; Matok, I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-analysis and Network Meta-analysis. Drug Saf. 2019, 42, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.H.; Shrestha, S.; Bjerregaard, L.G.; Ängquist, L.H.; Baker, J.L.; Jess, T.; Allin, K.H. Antibiotic exposure in early life and childhood overweight and obesity: A systematic review and meta-analysis. Diabetes. Obes. Metab. 2018, 20, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Simin, J.; Fornes, R.; Liu, Q.; Olsen, R.S.; Callens, S.; Engstrand, L.; Brusselaers, N. Antibiotic use and risk of colorectal cancer: A systematic review and dose-response meta-analysis. Br. J. Cancer 2020, 123, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Simin, J.; Tamimi, R.M.; Engstrand, L.; Callens, S.; Brusselaers, N. Antibiotic use and the risk of breast cancer: A systematic review and dose-response meta-analysis. Pharmacol. Res. 2020, 160, 105072. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnoletti, D.; Piani, F.; Cicero, A.F.G.; Borghi, C. The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature. J. Clin. Med. 2022, 11, 3557. https://doi.org/10.3390/jcm11123557
Agnoletti D, Piani F, Cicero AFG, Borghi C. The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature. Journal of Clinical Medicine. 2022; 11(12):3557. https://doi.org/10.3390/jcm11123557
Chicago/Turabian StyleAgnoletti, Davide, Federica Piani, Arrigo F. G. Cicero, and Claudio Borghi. 2022. "The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature" Journal of Clinical Medicine 11, no. 12: 3557. https://doi.org/10.3390/jcm11123557
APA StyleAgnoletti, D., Piani, F., Cicero, A. F. G., & Borghi, C. (2022). The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature. Journal of Clinical Medicine, 11(12), 3557. https://doi.org/10.3390/jcm11123557