Could Candida Overgrowth Be Involved in the Pathophysiology of Autism?
Abstract
:1. Introduction
2. Literature Search Strategy
2.1. Inclusion and Exclusion Criteria
2.2. Study Selection
3. Candida and Their Metabolites Isolated from Stool, Urine, andBlood Samples from Children with ASD
3.1. Candida Isolated from Stool Samples from Children with ASD
3.2. Candida Metabolites Isolated from Urine Samplesfrom Children with ASD
3.3. Candida Isolated from Blood Samples from Children with ASD
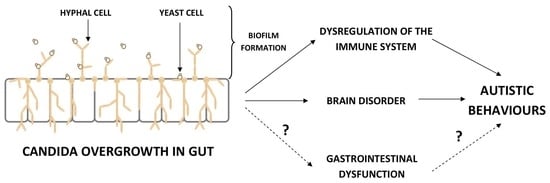
4. The Influence Candida Overgrowth on the Immune System, Brain, and Behavior of ASD Children—Mechanisms of Action
4.1. The Dysregulation of the Immune System
4.2. The Influence on Brain and Behavior in ASD Children
5. Treatment
5.1. Diet Intervention against Candida Overgrowth
5.2. Probiotic Intervention against Candida Overgrowth
5.3. Antifungal Agents against Candida Overgrowth
5.4. Fecal Microbiota Transplantation (FMT) and Microbiota Transfer Therapy (MTT) against Candida Overgrowth
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riglin, L.; Wootton, R.E.; Thapar, A.; Livingston, L.; Langley, K.; Collishaw, S.; Tagg, J.; Smith, G.D.; Stergiakouli, E.; Tilling, K.M.; et al. Variable emergence of autism spectrum disorder symptoms from childhood to early adulthood. Am. J. Psychiatry 2021, 178, 752–760. [Google Scholar] [CrossRef]
- Patterson, P.H. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011, 17, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Zerbo, O.; Qian, Y.; Yoshida, C.; Grether, J.K.; Van de Water, J.; Croen, L.A. Maternal infection during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2015, 45, 4015–4025. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhao, S.; Dalman, C.; Karlsson, H.; Gardner, R. Association of maternal diabetes with neurodevelopmental disorders: Autism spectrum disorders, attention-deficit/hyperactivity disorder and intellectual disability. Int. J. Epidemiol. 2021, 50, 459–474. [Google Scholar] [CrossRef]
- Amsyari, F.; Mukarromah, N. Reconsideration of early childhood vaccination (an epidemiological study on relationships between vaccination and autism). Folia Med. Indones. 2003, 39, 102–106. [Google Scholar]
- Blaylock, R.L. The danger of excessive vaccination during brain development. Med. Veritas 2008, 5, 1727–1741. [Google Scholar]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Horning, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, M.H.; Kumar, A.; Arora, S.; Akter, R. Role of metallic pollutants in neurodegeneration: Effects of aluminium, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ. Sci. Pollut. Res. 2021, 28, 8989–9001. [Google Scholar] [CrossRef]
- Rosen, N.J.; Yoshida, C.K.; Croen, L.A. Infection in the first 2 years of life and autism spectrum disorders. Pediatrics 2007, 119, e61–e69. [Google Scholar] [CrossRef]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Austin, D.W. Gastrointestinal microbiology in autistic spectrum disorder: A review. Rev. Med. Microbiol. 2010, 21, 44–50. [Google Scholar] [CrossRef]
- Bransfield, R.C. Preventable cases of autism: Relationship between chronic infectious diseases and neurological outcome. Pediatric Health 2009, 3, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Kovtun, A.S.; Averina, O.V.; Alekseeva, M.G.; Danilenko, V.N. Antibiotic resistance genes in the gut microbiota of children with autistic spectrum disorder as possible predictors of the disease. Microb. Drug Resist. 2020, 26, 1307–1320. [Google Scholar] [CrossRef]
- Champagne-Jorgensen, K.; Kunze, W.A.; Forsythe, P.; Bienenstock, J.; McVey Neufeld, K.A. Antibiotics and the nervous system: More than just the microbes? Brain Behav. Immun. 2019, 77, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, A.; Deonandan, R.; Konkle, A.T. The link between autism spectrum disorder and gut microbiota: A scoping review. Autism 2020, 24, 1328–1344. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Exp. Rev. Gastroenterol. Hepatol. 2021, 15, 599–622. [Google Scholar] [CrossRef]
- Lasheras, I.; Seral, P.; Latorre, E.; Barroso, E.; Gracia-García, P.; Santabárbara, J. Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian J. Psychiatry 2020, 47, 101874. [Google Scholar] [CrossRef]
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol.Nutr. 2010, 5, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Muthuirulan, P. Leaky Gut Syndrome: Mystery Illness Triggered by Candida albicans. J. Nutr. Health Food Eng. 2016, 4, 448–449. [Google Scholar] [CrossRef]
- Jyonouchi, H. Food allergy and autism spectrum disorders: Is there a link? Curr. Allergy Asthma. Rep. 2009, 9, 194–201. [Google Scholar] [CrossRef]
- Jyonouchi, H. Autism spectrum disorders and allergy: Observation from a pediatric allergy/immunology clinic. Exp. Rev. Clin. Immunol. 2010, 6, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, C.; Lama, A.; Lembo, F.; Mollica, M.P.; Calignano, A.; MattaceRaso, G. Interplay between peripheral and central inflammation in autism spectrum disorders: Possible nutritional and therapeutic strategies. Front. Physiol. 2018, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyonouchi, H.; Geng, L. Associations between monocyte and T cell cytokine profiles in autism spectrum disorders: Effects of dysregulated innate immune responses on adaptive responses to recall antigens in a subset of ASD children. Int. J. Mol. Sci. 2019, 20, 4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in autism spectrum disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Siniscalco, D.; Schultz, S.; Brigida, A.L.; Antonucci, N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doernberg, E.; Hollander, E. Neurodevelopmental disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 2016, 21, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ansary, A. GABA and glutamate imbalance in autism and their reversal as novel hypothesis for effective treatment strategy. Autism Dev. Disord. 2020, 18, 46–63. [Google Scholar] [CrossRef]
- Márquez-Caraveo, M.E.; Ibarra-González, I.; Rodríguez-Valentín, R.; Ramírez-García, M.Á.; Pérez-Barrón, V.; Lazcano-Ponce, E.; Vela-Amieva, M. Brief report: Delayed diagnosis of treatable inborn errors of metabolism in children with autism and other neurodevelopmental disorders. J. Autism Dev. Disord. 2021, 51, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Narbad, A.; Rodríguez, J.M. Autism spectrum disorder associated with gut microbiota at immune, metabolomic, and neuroactive level. Front. Neurosci. 2020, 14, 1072. [Google Scholar] [CrossRef]
- Guissart, C.; Latypova, X.; Rollier, P.; Khan, T.N.; Stamberger, H.; McWalter, K.; Cho, M.T.; Kjaergaard, S.; Weckhuysen, S.; Lesca, G.; et al. Dual molecular effects of dominant RORA mutations cause two variants of syndromic intellectual disability with either autism or cerebellar ataxia. Am. J. Hum. Genet. 2018, 102, 744–759. [Google Scholar] [CrossRef] [Green Version]
- Melke, J. Autism: Which genes are involved. Clin. Neuropsychiat. 2008, 5, 62–69. [Google Scholar]
- Williams, E.L.; Casanova, M.F.; Switala, A.E.; Li, H.; Qiu, M. Transposable elements occur more frequently in autism-risk genes: Implications for the role of genomic instability in autism. Translat. Neurosci. 2013, 4, 172–202. [Google Scholar] [CrossRef] [Green Version]
- Risch, N.; Hoffmann, T.J.; Anderson, M.; Croen, L.A.; Grether, J.K.; Windham, G.C. Familial recurrence of autism spectrum disorder: Evaluating genetic and environmental contributions. Am. J. Psychiatry 2014, 171, 1206–1213. [Google Scholar] [CrossRef]
- Eshraghi, R.S.; Deth, R.C.; Mittal, R.; Aranke, M.; Kay, S.I.S.; Moshiree, B.; Eshraghi, A.A. Early disruption of the microbiome leading to decreased antioxidant capacity and epigenetic changes: Implications for the rise in autism. Front. Cell Neurosci. 2018, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psych. 2017, 56, 466–474. [Google Scholar] [CrossRef] [Green Version]
- El-Ansary, A.; Bhat, R.S.; Zayed, N. Gut microbiome and sex bias in autism spectrum disorders. Curr. Behav. Neurosci. Rep. 2020, 7, 22–31. [Google Scholar] [CrossRef]
- Dhaliwal, K.K.; Orsso, C.E.; Richard, C.; Haqq, A.M.; Zwaigenbaum, L. Risk factors for unhealthy weight gain and obesity among children with autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 3285. [Google Scholar] [CrossRef] [Green Version]
- Noguera, M. Human gut microbiome metabolism and autism spectrum disorder. J. Agric. Life Sci. 2020, 7, 2. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Cox, S.B.; Meyer, D.; Austin, D.W. Molecular characterization of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012, 5, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S. What are the effects of proton pump inhibitors on the small intestine? World J. Gastroenterol. 2015, 21, 6817–6819. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012, 70, S10–S13. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef] [Green Version]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabro, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Finegold, S.M. Desulfovibrio species are potentially important in regressive autism. Med. Hypotheses 2011, 77, 270274. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Gómez-Fernández, A.; Chueca, N.; Torre-Aguilar, M.J.D.L.; Gil, Á.; Perez-Navero, J.L.; Flores-Rojas, K.; Martin-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism spectrum disorder (ASD) with and without mental regression is associated with changes in the fecal microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Amin Nordin, S.; Basir, R.; Abdullah, M. Mycobiome in the gut: A multiperspective review. Med. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdogan, A.; Rao, S.S. Small intestinal fungal overgrowth. Curr. Gastroenterol. Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Zou, R.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zheng, H. Dysbiosis of gut fungal microbiota in children with autism spectrum disorders. J. Autism Dev. Disord. 2021, 51, 267–275. [Google Scholar] [CrossRef]
- Tiew, P.Y.; Mac Aogain, M.; Ali, N.A.T.B.M.; Thang, K.X.; Goh, K.; Lau, K.J.; Chotirmall, S.H. The mycobiome in health and disease: Emerging concepts, methodologies and challenges. Mycopathologia 2020, 185, 207–231. [Google Scholar] [CrossRef]
- Pappas, P.G.; Rex, J.H.; Sobel, J.D.; Filler, S.G.; Dismukes, W.E.; Walsh, T.J.; Edwards, J.E. Guidelines for treatment of candidiasis. Clin. Inf. Dis. 2004, 38, 161–189. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.; Kang, D.W.; Adams, J.; Krajmalnik-Brown, R. Analysis of Yeast and Fungi in Children with ASD vs. Neurotypical Controls. Undergraduate Thesis, Honors College at Arizona State University, Tempe, AZ, USA, 2018. [Google Scholar]
- Shaw, W.; Baptist, J.; Geenens, D. Immunodeficiency, gastrointestinal Candidiasis, wheat and dairy sensitivity, abnormal urine arabinose, and autism: A case study. N. Am. J. Med. Sci. 2010, 3, 1. [Google Scholar] [CrossRef]
- Ahmed, S.A.S.; Meheissen, M.A.; Azouz, H.G.; Ashry, M.H.; Roshdy, Y.S.; Gad, H.A.; Ibrahim, A.E. Study of Candida species in stool of children with autism spectrum disorders in Alexandria, Egypt. Microbiol. Res. J. Int. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Austin, D.W. Faecal microbiota of individuals with autism spectrum disorder. E-J. Appl. Psychol. Clin. Soc. Issues 2010, 6, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 16, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarcioglu, A.S.; Kiraz, N.; Aydin, A. Microbiota–gut–brain axis: Yeast species isolated from stool samples of children with suspected or diagnosed autism spectrum disorders and in vitro susceptibility against nystatin and fluconazole. Mycopathologia 2016, 181, 1–7. [Google Scholar] [CrossRef]
- Jendraszak, M.; Gałęcka, M.; Kotwicka, M.; Regdos, A.; Pazgrat-Patan, M.; Andrusiewicz, M. Could selected gut microorganisms be diagnostic biomarkers for autism spectrum disorders? Study based on a commercial microbiota test. Res. Square 2021, 1–19. [Google Scholar] [CrossRef]
- Emam, A.M.; Mamdouh, E.; Abdelrahim, S. Candida albicans infection in autism. J. Am. Sci. 2012, 8, 739–744. [Google Scholar]
- El-Shouny, W.A.; Ismail, S.; Elzawawy, N.; Hegazy, S. Efficacy of herbal control of the yeasts isolated from autistic children. GJBAHS 2016, 5, 65–73. [Google Scholar]
- Lo, H.J.; Kohler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Carlisle, P.L.; Banerjee, M.; Lazzell, A.; Monteagudo, C.; López-Ribot, J.L.; Kadosh, D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc. Natl. Acad. Sci. USA 2009, 106, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toenjes, K.A.; Munsee, S.M.; Ibrahim, A.S.; Jeffrey, R.; Edwards, J.E., Jr.; Johnson, D.I. Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 2005, 49, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Yosef, H.; Vivanco Gonzalez, N.; Ben-Aroya, S.; Kron, S.J. Chemical inhibitors of Candida albicans hyphal morphogenesis target endocytosis. Sci. Rep. 2017, 7, 5692. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.L.; Padovan, A.C.B.; Chaves, G.M. Current knowledge of Trichosporon spp. and Trichosporonosis. Clin. Microbiol. Rev. 2011, 24, 682–700. [Google Scholar] [CrossRef] [Green Version]
- Laswi, I.; Shafiq, A.; Al-Ali, D.; Burney, Z.; Pillai, K.; Salameh, M.; Mhaimeed, N.; Zakaria, D.; Chaari, A.; Yousri, N.A.; et al. A comparative pilot study of bacterial and fungal dysbiosis in neurodevelopmental disorders and gastrointestinal disorders: Commonalities, specificities and correlations with lifestyle. Microorganisms 2021, 9, 741. [Google Scholar] [CrossRef]
- Shaw, W.; Kassen, E.; Chaves, E. Increased urinary excretion of analogs of Krebs cycle metabolites and arabinose in two brothers with autistic features. Clin. Chem. 1995, 41, 1094–1104. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Błaszczyk, S. The level of arabinitol in autistic children after probiotic therapy. Nutrients 2012, 28, 124–126. [Google Scholar] [CrossRef]
- Noto, A.; Fanos, V.; Barberini, L.; Grapov, D.; Fattuoni, C.; Zaffanello, M.; Casanova, A.; Fenu, G.; De Giacomo, A.; De Angelis, M.; et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J.Matern.-Fetal Neonatal Med. 2014, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Burdette, C.h.K.; Bralley, J.A. Urinary markers of yeast overgrowth. Integ. Med. 2004, 3, 24–29. [Google Scholar]
- Becker, J.; Boles, E. A modified Saccharomyces cerevisiae strain that consumes L-Arabinose and produces ethanol. Appl. Environ. Microbiol. 2003, 69, 4144–4150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, P.; Verho, R.; Putkonen, M.; Londesborough, J.; Penttila, M. Production of ethanol from L-arabinose by Saccharomyces cerevisiae containing a fungal L-arabinose pathway. FEM Yeast Res. 2003, 3, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, D.; Weinstock, A.; Antharam, V.C.; Gu, H.; Jasbi, P.; Shi, X.; Dirks, B.; Krajmalnik-Brown, R.; Maldonado, J.; Guinan, J.; et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol. Ecol. 2020, 96, fiz187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, R.S. Urinary markers of intestinal yeast. Townsend Lett. Dr. Patients 2003, 245, 96–97. [Google Scholar]
- Fonseca, A. Utilization of tartaric acid and related compounds by yeasts: Taxonomic implications. Can. J. Microbiol. 1992, 38, 1242–1251. [Google Scholar] [CrossRef]
- Fonseca, A.; Fell, J.W.; Kurtzman, C.P.; Spencer-Martins, I. Candida tartarivorans sp. nov., an anamorphic ascomycetous yeast with the capacity to degrade L(+)- and meso-tartaric acid. Int. J. Syst.Evol. Microbiol. 2000, 50, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Markova, N. Dysbiotic microbiota in autistic children and their mothers: Persistence of fungal and bacterial wall-deficient L-form variants in blood. Sci. Rep. 2019, 9, 13401. [Google Scholar] [CrossRef] [Green Version]
- Hughes, H.K.; Ashwood, P. Anti-Candida albicans IgG antibodies in children with autism spectrum disorders. Front. Psychiatry 2018, 9, 627. [Google Scholar] [CrossRef]
- Janeway, C.J.; Travers, P.; Walport, M. The Distribution and Functions of Immunoglobulin Isotypes. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Rabalais, G.P.; Samiec, T.D.; Bryant, K.K.; Lewis, J. Invasive candidiasis in infants weighing more than 2500 grams at birth admitted to a neonatal intensive care unit. Pediatr. Infect. Dis. J. 1996, 15, 348–352. [Google Scholar] [CrossRef]
- Rimland, B. Candida-caused autism. Autism Res. Rev.Inern. 1988, 2, 3. [Google Scholar]
- Crook, W.G. Yeast can affect behavior and learning. Acad.Ther. 1984, 19, 517–526. [Google Scholar] [CrossRef]
- Carter, C.J. Autism genes and the leukocyte transcriptome in autistic toddlers relate to pathogen interactomes, infection and the immune system. A role for excess neurotrophic sAPPα and reduced antimicrobial Aβ. Neurochem. Int. 2019, 126, 36–58. [Google Scholar] [CrossRef]
- Colina, A.R.; Aumont, F.; Deslauriers, N.; Belhumeur, P.; de Repentigny, L. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Inf. Immun. 1996, 64, 4514–4519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noverr, M.C.; Huffnagle, G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004, 72, 6206–6210. [Google Scholar] [CrossRef] [Green Version]
- García, C.; Tebbji, F.; DaigneaultMLiu, N.N.; Kohler, J.R.; Allen-Vercoe, E.; Sellam, A. The human gut microbial metabolome modulates fungal growth via the TOR signalling pathway. mSphere 2017, 2, e00555-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.L.; Limon, J.J.; Underhill, D.M. Immunity to commensal fungi: Detente and disease. Annu. Rev. Pathol. 2017, 12, 359–385. [Google Scholar] [CrossRef]
- Roth, S.; Ruland, J. Caspase recruitment domain-containing protein 9 signalling in innate immunity and inflammation. Trends Immunol. 2013, 34, 243–250. [Google Scholar] [CrossRef]
- Czakai, K.; Leonhardt, I.; Dix, A.; Bonin, M.; Linde, J.; Einsele, H.; Kurzai, O.; Loeffler, J. Kruppel-like Factor 4 modulates interleukin-6 release in human dendritic cells after in vitro stimulation with Aspergillus fumigatus and Candida albicans. Sci. Rep. 2016, 6, 27990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, H.R.; Gaffen, S.L. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J. Immunol. 2015, 195, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorlag, S.; Roring, R.J.; Joosten, L.A.B.; Netea, M.G. The role of the interleukin-1 family in trained immunity. Immunol. Rev. 2018, 281, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J.; Krigsman, A.; Jepson, B.; Wakefield, A. Low serum myeloperoxidase in autistic children with gastrointestinal disease. Clin. Exp. Gastroenterol. 2009, 2, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, R.I.; Cline, M.J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: The role of myeloperoxidase in resistance to Candida infection. J. Clin.Investig. 1969, 48, 1478–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanza, F. Clinical manifestation of myeloperoxidase deficiency. J. Mol. Med. 1998, 76, 676–681. [Google Scholar] [CrossRef]
- Ceylan, M.F.; Hesapcioglu, S.T.; Yavas, C.P.; Senat, A.; Erel, O. Serum ischemia-modified albumin levels, myeloperoxidase activity and peripheral blood mononuclear cells in autism spectrum disorder (ASD). J. Autism Dev. Disord. 2012, 51, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Topal, Z.; Tufan, A.E.; Karadag, M.; Gokcen, C.; Akkaya, C.; Sarp, A.S.; Bahsi, I.; Kilinc, M. Evaluation of peripheral inflammatory markers, serum B12, folate, ferritin levels and clinical correlations in children with autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD). Nordic. J. Psychiatry 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Stanzani, M.; Orciuolo, E.; Lewis, R.; Kontoyiannis, D.P.; Martins, S.L.; St John, L.S.; Komanduri, K.V. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005, 105, 2258–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubmann, R.; Hilgarth, M.; SchnablSPonath, E.; Reiter, M.; Demirtas, D.; Sieghart, W.; Valent, P.; Zielinski Ch Jager, U.; Shehata, M. Gliotoxin is a potent NOTCH 2 transactivation inhibitor and efficiently induces apoptosis in chronic lymphocytic leukaemia (CLL) cells. Br. J. Haematol. 2013, 160, 618–629. [Google Scholar] [CrossRef]
- Shah, D.; Larsen, B. Clinical isolates of yeast produce a gliotoxin-like substance. Mycopathologia 1991, 116, 203–208. [Google Scholar] [CrossRef]
- Podzorski, R.; Herron, M.; Fast, D.; Nelson, R. Pathogenesis of candidiasis immunosuppression by cell wall mannan catabolites. Arch. Surg. 1989, 124, 1290–1294. [Google Scholar] [CrossRef]
- Witkin, S.S. Defective immune responses in patients with recurrent candidiasis. Infect. Med. 1985, 129–132. [Google Scholar]
- Ho, J.; Yang, X.; Nikou, S.A.; Kichik, N.; Donkin, A.; Ponde, N.O.; Richardson, J.P.; Gratacap, R.L.; Archambault, L.S.; Zwirner, C.P.; et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat. Commun. 2019, 10, 2297. [Google Scholar] [CrossRef] [Green Version]
- Naglik, J.R.; Gaffen, S.L.; Hube, B. Candidalysin: Discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019, 52, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [Green Version]
- Kasper, L.; Konig, A.; Koenig, P.A.; Gresnigt, M.S.; Westman, J.; Drummond, R.A.; Lionakis, M.S.; Gross, O.; Ruland, J.; Naglik, J.R.; et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018, 9, 4260. [Google Scholar] [CrossRef]
- Rogiers, O.; Frising, U.C.; Kucharikova, S.; Jabra-Rizk, M.A.; van Loo, G.; Van Dijck, P.; Wullaert, A. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. MBio 2019, 10, e02221-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonhardt, I.; Spielberg, S.; Weber, M.; Albrecht-Eckardt, D.; Bläss, M.; Claus, R.; Barz, D.; Scherlach, K.; Hertweck, C.; Löffler, J.; et al. The fungal quorum-sensing molecule farnesol activates innate immune cells but suppresses cellular adaptive immunity. mBio 2015, 6, e00143-15. [Google Scholar] [CrossRef] [Green Version]
- Noverr, M.C.; Phare, S.M.; Toews, G.B.; Coffey, M.J.; Huffnagle, G.B. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 2001, 69, 2957–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.G.; Udayanga, K.G.; Totsuka, N.; Weinberg, J.B.; Nunez, G.; Shibuya, A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE(2). Cell Host Microbe 2014, 15, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.G.; Lim, Y.S.; Tan, A.; Leong, R.; Pavelka, N. Fungal symbionts produce prostaglandin E2 to promote their intestinal colonization. Front. Cell Infect. Microbiol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrus, C.J. A biochemical rationale for the interaction between gastrointestinal yeast and autism. Med. Hypothesis 2012, 79, 784–785. [Google Scholar] [CrossRef]
- Denaro, F.J.; López-Ribot, J.L.; Chaffin, W.L. Adhesion of Candida albicans to brain tissue of Macaca mulatta in an ex vivo assay. Infect. Immun. 1995, 63, 3438–3441. [Google Scholar] [CrossRef] [Green Version]
- Jong, A.Y.; Stins, M.F.; Huang, S.H.; Chen, S.H.M.; Kim, K.S. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect Immun 2001, 69, 4536–4544. [Google Scholar] [CrossRef] [Green Version]
- Navarathna, D.H.; Munasinghe, J.; Lizak, M.J.; Nayak, D.; McGavern, D.B.; Roberts, D.D. MRI confirms loss of blood–brain barrier integrity in a mouse model of disseminated candidiasis. NMR Biomed. 2013, 26, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Ophelders, D.R.; Gussenhoven, R.; Lammens, M.; Küsters, B.; Kemp, M.W.; Newnham, J.P.; Payne, M.S.; Kallapur, S.G.; Jobe, A.H.; Zimmermann, L.J.; et al. Neuroinflammation and structural injury of the fetal ovine brain following intra-amniotic Candida albicans exposure. J. Neuroinflamm. 2016, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Bochenek, J.; Król, K.; Krawczyńska, A.; Antushevich, H.; Pawlina, B.; Herman, A.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central Interleukin-1β suppresses the nocturnal secretion of melatonin. Med. Inflamm. 2016, 2016, 2589483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skelly, D.T.; Griffin, E.W.; Murray, C.L.; Harney, S.; O’Boyle, C.; Hennessy, E.; Dansereau, M.A.; Nazmi, A.; Tortorelli, L.; Rawlins, J.N.; et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol. Psychiatry 2019, 24, 1533–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, J.S.; Camilli, G.; Kotowicz, N.K.; Ho, J.; Richardson, J.P.; Naglik, J.R. Role for IL-1 family cytokines in fungal infections. Front. Microbiol. 2021, 12, 633047. [Google Scholar] [CrossRef]
- Reichelt, K.; Knivsberg, A. The possibility and probability of a gut-to-brain connection in autism. Am. Clin. Psychiatry 2009, 21, 205–211. [Google Scholar]
- Dhossche, D.; Applegate, H.; Abraham, A.; Maertens, P.; Bland, L.; Bencsath, A.; Martinez, J. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: Stimulus for a GABA hypothesis of autism. Med. Sci. Monitor 2002, 8, PR1–PR6. [Google Scholar]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Thuras, P.D. GABA A receptor downregulation in brains of subjects with autism. J. Autism Dev. Disord. 2009, 39, 223. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.L.; Anacker, A.M.; Veenstra-Vander Weele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Connors, S.L.; Matteson, K.J.; Sega, G.A.; Lozzio, C.B.; Carroll, R.C.; Zimmerman, A.W. Plasma serotonin in autism. Pediatr. Neurol. 2006, 35, 182–186. [Google Scholar] [CrossRef]
- Cook, E.H.; Leventhal, B.L. The serotonin system in autism. Curr. Opin. Pediatr. 1996, 8, 348–354. [Google Scholar] [CrossRef]
- Marler, S.; Ferguson, B.J.; Lee, E.B.; Peters, B.; Williams, K.C.; McDonnell, E.; Macklin, E.A.; Levitt, P.; Gillespie, C.H.; Anderson, G.M.; et al. Brief report: Whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J. Autism Dev. Disord. 2016, 46, 1124–1130. [Google Scholar] [CrossRef] [Green Version]
- Doenyas, C. Dietary interventions for autism spectrum disorder: New perspectives from the gut-brain axis. Physiol. Behav. 2018, 194, 577–582. [Google Scholar] [CrossRef]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: A systematic review. Psychiatry Res. 2020, 2020, 113471. [Google Scholar] [CrossRef]
- Volkert, V.M.; Vaz, P.C.M. Recent studies on feeding problems in children with autism. J. Appl.Behav. Anal. 2010, 43, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, W.G.; Berry, R.C.; McCracken, C.; Nuhu, N.N.; Marvel, E.; Saulnier, C.A.; Klin, A.; Jones, W.; Jaquess, D.L. Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. J. Autism Dev. Disord. 2013, 43, 2159–2173. [Google Scholar] [CrossRef]
- Vissoker, R.E.; Latzer, Y.; Gal, E. Eating and feeding problems and gastrointestinal dysfunction in Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2015, 12, 10–21. [Google Scholar] [CrossRef]
- Lam, S.; Zuo, T.; Ho, M.; Chan, F.K.L.; Chan, P.K.S.; Ng, S.C. Review article: Fungal alterations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2019, 50, 1159–1171. [Google Scholar] [CrossRef]
- Pan, C.; Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PloS ONE 2013, 8, e66019. [Google Scholar]
- Jacobs, G.; Kjaer, J. Beat Candida through Diet: A Complete Dietary Programme for Suffers of Candidiasis; Random House: New York, NY, USA, 2012. [Google Scholar]
- Romeo, M.G.; Romeo, D.M.; Trovato, L.; Oliveri, S.; Palermo, F.; Cota, F.; Betta, P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2011, 31, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wan, G.B.; Huang, M.S.; Agyapong, G.; Zou, T.L.; Zhang, X.Y.; Liu, Y.W.; Song, Y.Q.; Tsai, Y.C.; Kong, X.J. Probiotic therapy for treating behavioral and gastrointestinal symptoms in autism spectrum disorder: A systematic review of clinical trials. Curr. Med. Sci. 2019, 39, 173–184. [Google Scholar] [CrossRef]
- MacAlpine, J.; Daniel-Ivad, M.; Liu, Z.; Yano, J.; Revie, N.M.; Todd, R.T.; Stogios, P.J.; Sanchez, H.; O’Meara, T.R.; Tompkins, T.A.; et al. A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 2021, 12, 6151. [Google Scholar] [CrossRef]
- Murzyn, A.; Krasowska, A.; Stefanowicz, P.; Dziadkowiec, D.; Łukaszewicz, M. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS ONE 2010, 5, e12050. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, W.; Kassen, E.; Chaves, E. Assessment of antifungal drug therapy in autism by measurement of suspected microbial metabolites in urine with gas chromatography-mass spectrometry. Clin. Pract. Altern. Med. 2000, 1, 15–26. [Google Scholar]
- Zimmerman, B.; Weber, E. Candida and “20th-century disease”. CMAJ Can. Med. Assoc. J. 1985, 133, 965. [Google Scholar]
- Shareck, J.; Belhumeur, P. Modulation of morphogenesis in Candida albicans by various small molecules. Eukaryotic Cell 2011, 10, 1004–1012. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Kung, J.Y.; Tun, H.M.; Wine, E.; Madsen, K.L.; Zwaigenbaum, L.; Haqq, A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res. 2021, 14, 1820–1836. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Akagawa, G.; Abe, S.; Yamaguchi, H. Mortality of Candida albicans-infected mice is facilitated by superinfection of Escherichia coli or administration of its lipopolysaccharide. J. Infect. Dis. 1995, 171, 1539–1544. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, X.; Xi, L.; Shi, Y.; Peng, L.; Wang, C.; Zou, L.; Yang, Y. Mo1667 fecal microbiota transplantation for children with an autism spectrum disorder. Gastrointest. Endoscop. 2019, 89, AB512–AB513. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.F.; et al. Fungal trans-kingdom dynamics linked to responsiveness to fecal microbiota transplantation (FMT) therapy in ulcerative colitis. Cell Host Microbe 2020, 27, 823–829. [Google Scholar] [CrossRef]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.J.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018, 67, 1920–1941. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.W.; Phelps, E.; Ganapini, V.; Khan, N.; Ouyang, F.; Xu, H.; Khanna, S.; Tariq, R.; Friedman-Moraco, R.J.; Woodworth, M.H.; et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: A multicenter experience. Am. J. Transplant. 2019, 19, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Johnson, S. Fecal microbiota transplantation (FMT) for C. difficile infection, just say ‘No’. Anaerobe 2019, 60, 102092. [Google Scholar] [CrossRef]

| Study Groups | Candida sp. Identified in a Stool Samples | Statistical Significance (ASD vs. Control) | Impact on Children with ASD | Ref. |
|---|---|---|---|---|
| Candida | Not statistically significant |
| [55] |
| ||||
| ||||
| C. parapsilosis | No data |
| [56] |
| ||||
| C. glabrata C. parapsilosis | Not statistically significant |
| [57] |
| C. tropicalis |
| |||
| C. albicans | ||||
| C. krusei | ||||
| Candida | Not statistically significant |
| [58] |
| ||||
| Candida | Partially significant |
| [46] |
| Candida | Statistically significant |
| [59] |
| ||||
| ||||
| ||||
| ||||
| Candida | Not statistically significant |
| [60] |
| C. albicans C.krusei C. glabrata | No data |
| [61] |
| Candida | Not statistically significant |
| [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, A.; Herman, A.P. Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? J. Clin. Med. 2022, 11, 442. https://doi.org/10.3390/jcm11020442
Herman A, Herman AP. Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? Journal of Clinical Medicine. 2022; 11(2):442. https://doi.org/10.3390/jcm11020442
Chicago/Turabian StyleHerman, Anna, and Andrzej Przemysław Herman. 2022. "Could Candida Overgrowth Be Involved in the Pathophysiology of Autism?" Journal of Clinical Medicine 11, no. 2: 442. https://doi.org/10.3390/jcm11020442
APA StyleHerman, A., & Herman, A. P. (2022). Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? Journal of Clinical Medicine, 11(2), 442. https://doi.org/10.3390/jcm11020442