A Machine Learning Model for the Accurate Prediction of 1-Year Survival in TAVI Patients: A Retrospective Observational Cohort Study
Abstract
:1. Introduction
2. Methods
Statistical Methods
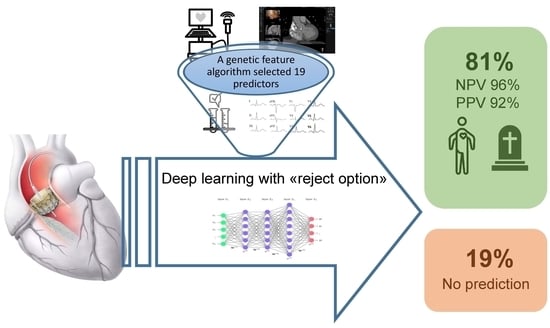
3. Results
4. Discussion
5. Strengths and Limitations of This Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J.; Modine, T.; Prendergast, B.; Praz, F.; Pilgrim, T.; Petrinic, T.; Nikolakopoulou, A.; Salanti, G.; et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Isogai, T.; Agrawal, A.; Shekhar, S.; Puri, R.; Reed, G.W.; Yun, J.J.; Unai, S.; Burns, D.J.; Vargo, P.R.; et al. Feasibility and Safety of Same-Day Discharge Following Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2022, 15, 575–589. [Google Scholar] [CrossRef]
- Goel, A.; Malik, A.H.; Bandyopadhyay, D.; Chakraborty, S.; Gupta, R.; Abbott, J.D.; Ahmad, H. The 30-Day Readmission Rate of Same-Day Discharge Following Transcatheter Aortic Valve Implantation (from National Readmission Database 2015 to 2019). Am. J. Cardiol. 2022, 176, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Barbanti, M.; Picci, A.; Todaro, D.; La Spina, K.; Di Simone, E.; D’arrigo, P.; Criscione, E.; Valvo, R.; Reddavid, C.; et al. Predictors and safety of next-day discharge in patients undergoing transfemoral transcatheter aortic valve implantation. Eurointervention 2020, 16, e494–e501. [Google Scholar] [CrossRef]
- Pollari, F.; Dell’Aquila, A.M.; Söhn, C.; Marianowicz, J.; Wiehofsky, P.; Schwab, J.; Pauschinger, M.; Hitzl, W.; Fischlein, T.; Pfeiffer, S. Risk factors for paravalvular leak after transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2019, 157, 1406–1415.e3. [Google Scholar] [CrossRef]
- Pollari, F.; Großmann, I.; Vogt, F.; Kalisnik, J.M.; Cuomo, M.; Schwab, J.; Fischlein, T.; Pfeiffer, S. Risk factors for atrioventricular block after transcatheter aortic valve implantation: A single-centre analysis including assessment of aortic calcifications and follow-up. Europace 2019, 21, 787–795. [Google Scholar] [CrossRef]
- Pollari, F.; Hitzl, W.; Vogt, F.; Cuomo, M.; Schwab, J.; Söhn, C.; Kalisnik, J.M.; Langhammer, C.; Bertsch, T.; Fischlein, T.; et al. Aortic valve calcification as a risk factor for major complications and reduced survival after transcatheter replacement. J. Cardiovasc. Comput. Tomogr. 2020, 14, 307–313. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Re-porting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; ESC Scientific Document Group; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744; discussion 744–745. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012, 33, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, U.; Jain, L.C. Feature Selection for Data and Pattern Recognition; Springer Publishing Company, Incorporated: Berlin/Heidelberg, Germany, 2014; pp. 191–192. [Google Scholar]
- Bishop, C.M. Neural Networks for Pattern Recognition; Oxford University Press Inc.: New York, NY, USA, 1995. [Google Scholar]
- Wolfram Research, Inc. Mathematica, Version 13.1; Wolfram Research, Inc.: Champaign, IL, USA, 2022. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; ESC/EACTS Scientific Document Group; et al. 2021 ESC/EACTS Guidelines for the man-agement of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, M.; Serruys, P.W. Transcatheter Aortic Valve Implantation in Lower-Risk Patients with Aortic Stenosis: Is It Justified to Be the Preferred Treatment? Circ. Cardiovasc. Interv. 2016, 9, e002944. [Google Scholar] [CrossRef] [PubMed]
- Babaliaros, V.; Devireddy, C.; Lerakis, S.; Leonardi, R.; Iturra, S.A.; Mavromatis, K.; Leshnower, B.G.; Guyton, R.A.; Kanitkar, M.; Keegan, P.; et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): Outcomes and cost analysis. JACC Cardiovasc. Interv. 2014, 7, 898–904. [Google Scholar] [CrossRef]
- Spence, M.S.; Baan, J.; Iacovelli, F.; Martinelli, G.L.; Muir, D.F.; Saia, F.; Bortone, A.S.; Densem, C.G.; Owens, C.G.; van der Kley, F.; et al. Prespecified Risk Criteria Facilitate Adequate Discharge and Long-Term Outcomes After Transfemoral Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2020, 9, e016990. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.F.; Kim, Y.; Villablanca, P.; Gupta, T.; Wiley, J.; Nieves-Rodriguez, B.G.; Rodriguez-Maldonado, J.; Maldonado, R.F.; Sant’Ana, I.D.L.; Sanina, C.; et al. Machine Learning Prediction Models for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.; Pilz, M.; Reich, C.; Leuschner, F.; Konstandin, M.; Katus, H.A.; Meder, B. Machine learning-based risk prediction of intrahospital clinical outcomes in patients undergoing TAVI. Clin. Res. Cardiol. 2021, 110, 343–356. [Google Scholar] [CrossRef] [PubMed]
- D’ascenzo, F.; Salizzoni, S.; Saglietto, A.; Cortese, M.; Latib, A.; Franzone, A.; Barbanti, M.; Nietlispach, F.; Holy, E.W.; Burriesci, G.; et al. Incidence, predictors and cerebrovascular consequences of leaflet thrombosis after transcatheter aortic valve implantation: A systematic review and meta-analysis. Eur. J. Cardio-Thoracic Surg. 2019, 56, 488–494. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; PARTNER 2 Investigators; et al. Transcatheter or Surgical Aortic-Valve Replacement in Inter-mediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Nazif, T.M.; Chen, S.; George, I.; Dizon, J.M.; Hahn, R.T.; Crowley, A.; Alu, M.C.; Babaliaros, V.; Thourani, V.H.; Herrmann, H.C.; et al. New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: An analysis from the PARTNER II trial. Eur. Heart J. 2019, 40, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Zeymer, U.; Clark, A.L.; Barrios, V.; Damy, T.; Drożdż, J.; Fonseca, C.; Kalus, S.; Ferber, P.C.; Koch, C.; et al. Association between sacubitril/valsartan initiation and changes in left ventricular ejection fraction: Insights from ARIADNE registry. Int. J. Cardiol. 2023, 370, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Dauw, J.; Sokolski, M.; Middleton, J.T.; Nijst, P.; Dupont, M.; Forouzan, O.; Rothman, A.M.; Ruschitzka, F.; Flammer, A.J.; Mullens, W. Ambulatory haemodynamic-guided management reduces heart failure hospitalizations in a multicentre European heart failure cohort. ESC Heart Fail. 2022, 9, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef]
- Engelman, D.T.; Crisafi, C.; Germain, M.; Greco, B.; Nathanson, B.H.; Engelman, R.M.; Schwann, T.A. Using urinary biomarkers to reduce acute kidney injury following cardiac surgery. J. Thorac. Cardiovasc. Surg. 2020, 160, 1235–1246.e2. [Google Scholar] [CrossRef] [PubMed]
- Flores-Umanzor, E.; Nogic, J.; Cepas-Guillén, P.; Hascoet, S.; Pysz, P.; Baz, J.A.; Cruz-González, I.; Amat-Santos, I.J.; Antúnez-Muiños, P.; González, J.C.; et al. Percutaneous paravalvular leak closure after transcatheter aortic valve implantation: The international PLUGinTAVI Registry. Eurointervention 2023, 19, e442–e449. [Google Scholar] [CrossRef]
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; COAPT Investigators; et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N. Engl. J. Med. 2023, 388, 2037–2048. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Mean or n (%) |
|---|---|
| Gender (male/female) | 45%/55% |
| Age, y (mean, range) | 81.9 (53.8–94.5) |
| Body mass index, kg/m2 (mean, range) | 27.1 (16.6–45.8) |
| Extracardiac arteriopathy | 109 (19%) |
| Prior cardiac surgery | 82 (15%) |
| Recent myocardial infarction | 131 (23%) |
| Prior PCI | 164 (29%) |
| Dialysis, n (%) | 11 (1.7%) |
| EuroSCORE II (mean ± SD) | 7% (±7) |
| Coronary obstruction | 5 (0.9%) |
| Ischemic stroke | 10 (1.8%) |
| Life-threatening bleeding | 12 (2.1%) |
| Major vascular complications | 29 (5.1%) |
| Need for permanent pacemaker implantation | 44 (7.8%) |
| Procedural mortality, n (%) | 7 (1.2%) |
| In-hospital mortality, n (%) | 24 (4.2%) |
| ICU stay in days (mean, range) | 2.0 (0–43) |
| Hospital stay in days (mean, range) | 10.25 (0–91) |
| Follow-up time in days (mean, range) | 927.1 (0–2665) |
| 1-year mortality, n (%) | 67 (10.2%) |
| Mean | Mean | Std | Std | ||
|---|---|---|---|---|---|
| Predictor | Survived within 1 Year | Died within 1 Year | Survived within 1 Year | Died within 1 Year | p-Value |
| Continuous Variable | |||||
| Age (years) | 82.2 | 80 | 5.6 | 8.29 | 0.0006 |
| Indexed effective orifice area | 0.4 | 0.4 | 0.09 | 0.1 | 0.18 |
| Body mass index | 27.2 | 26.7 | 4.77 | 4.76 | 0.45 |
| Body surface area | 1.8 | 1.8 | 0.22 | 0.25 | 0.54 |
| Estimated creatinine clearance (mL/min) | 48.1 | 44.7 | 18.71 | 22.11 | 0.24 |
| Creatinine (mg/dL) postprocedural peak | 1.26 | 1.76 | 0.80 | 1.06 | 0.000006 |
| Serum C-reactive protein (mg/dL) | 0.7 | 1.9 | 1.42 | 3.13 | 0.0003 |
| CT-based aortic annulus perimeter (mm) | 76.5 | 78.8 | 7.4 | 8.7 | 0.019 |
| CT-based aortic annulus area (mm2) | 4.5 | 4.8 | 0.88 | 1.04 | 0.008 |
| DLZ calcium load (mm3) | 879.9 | 903.9 | 630.24 | 672.27 | 0.78 |
| Eccentricity index | 0.2 | 0.2 | 0.07 | 0.07 | 0.9 |
| Baseline transvalvular Dmax | 76.8 | 69.8 | 24.06 | 26.34 | 0.049 |
| Baseline transvalvular Dmean | 45.4 | 42.2 | 14.43 | 17.56 | 0.17 |
| Baseline LV ejection fraction | 54.3 | 49.2 | 12.06 | 14.32 | 0.0015 |
| Baseline hemoglobin (g/dL) | 12.3 | 11.8 | 1.74 | 1.96 | 0.016 |
| Baseline hematocrit (%) | 37.2 | 36.1 | 4.81 | 5.47 | 0.13 |
| Grade of oversizing (%) | 0.2 | 0.1 | 0.18 | 0.23 | 0.53 |
| Platelet count (×1000/uL) | 219.3 | 227.9 | 69.66 | 92.25 | 0.47 |
| PR interval (ms) | 173.8 | 179.9 | 40.88 | 36.56 | 0.38 |
| QRS duration (ms) | 99 | 108 | 24.16 | 29.36 | 0.008 |
| QTc interval (ms) | 447.5 | 465.7 | 35.72 | 46.11 | 0.005 |
| SAPS2 | 25.9 | 31.1 | 10.98 | 14.22 | 0.0006 |
| Calcium load of aortic valve (mm3) | 815.7 | 814.6 | 583.85 | 649.9 | 0.99 |
| Calcium load of LVOT (mm3) | 64.2 | 89.3 | 101.72 | 113.37 | 0.09 |
| White blood cell count (×1000/uL) | 7.1 | 7.9 | 2.29 | 3.08 | 0.033 |
| Discharge transvalvular Dmean | 11.1 | 9.6 | 4.67 | 3.89 | 0.039 |
| Categorical Variable | |||||
| Survived within 1 Year | Died within 1 Year | ||||
| Gender (M/F) | 45%, 55% | 57%, 43% | 0.066 | ||
| Extracardiac arteriopathy | 17.8% | 30.3% | 0.016 | ||
| Non-insulin-dependent diabetes mellitus | 28.1% | 41.8% | 0.02 | ||
| New York Heart Association class (I, II, III, IV) | 0.8%, 11.2%, 74.5%, 13.6% | 1.5%, 7.6%, 65.2%, 25.7% | 0.053 | ||
| Baseline aortic valve insufficiency † | 25%, 58.7%, 15.8%, 0.5% | 30.9%, 49.1%, 16.4%, 3.6% | 0.057 | ||
| Baseline mitral valve insufficiency † | 13.1%, 64.9%, 21.1%, 0.9% | 5.7%, 56.7%, 22.5%, 15.1% | <0.0001 | ||
| Baseline tricuspid valve insufficiency † | 45.4%, 42.3%, 9.4%, 2.9% | 52.6%, 29.9%, 8.8%, 8.8% | 0.061 | ||
| Persistent/permanent atrial fibrillation | 29.9% | 41.8% | 0.067 | ||
| Valve prosthesis (type) § | 0%, 20.1%, 4.8%, 3.8%, 3.2%, 21.5%, 46.6% | 1.5%, 17.9%, 10.5%, 7.5%, 3%, 17.9%, 41.7% | 0.052 | ||
| Valve prosthesis’s label size ‡ | 0%, 34.7%, 8.9%, 34.1%, 4.8%, 12.5%, 3.4%, 1.4% | 1.5%, 26.9%, 6%, 29.8%, 4.5%, 22.4%, 7.4%, 1.5% | 0.04 | ||
| Valvuloplasty prior to prosthesis implantation | 98.4% | 94.3% | 0.02 | ||
| Postprocedural paravalvular regurgitation † | 73.6%, 21.9%, 4.5%, 0% | 70.8%, 18.8%, 8.3%, 2.1% | 0.016 | ||
| Model Performance | Support Vector Machine | Nearest Neighbour | Neuronal Network | Bayes Classifier | Random Forest |
|---|---|---|---|---|---|
| AUC | 74% | 72% | 78% | 82% | 81% |
| NPV/PPV | 88%/— 1 | 87%/— | 91%/71% | 90%/81% | 93%/— |
| Negative Predictive Power | Positive Predictive Power | Unpredicted | Total Correctly Predicted | |
|---|---|---|---|---|
| Training sample (10-fold cross-validation) | (305/315) 98% | (11/12) 92% | (68/395) 17% | (316/325) 97% |
| Test sample | (121/129) 94% | (0/0) - | (41/170) 24% | (121/129) 94% |
| Overall sample | (426/444) 96% | (11/12) 92% | (109/565) 19% | (437/456) 96% |
| ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 83.2 | 82.1 | 80.1 | 79.0 | 79.0 | 82.8 | 84.6 | 82.2 |
| Hemoglobin (g/dL) | 14.2 | 12.1 | 11.8 | 14.1 | 12.3 | 12.8 | 12.6 | 12.6 |
| White blood cell count (×1000/uL) | 6.9 | 7.3 | 4.5 | 6.0 | 7.0 | 6.9 | 6 | 5.7 |
| C-reactive protein (mg/dL) | 0.0 | 0.0 | 0.6 | 0.4 | 0.5 | 2.6 | 0.5 | 0.5 |
| Baseline left ventricular ejection fraction | 60 | 75 | 30 | 60 | 60 | 62 | 55 | 55 |
| QRS duration (ms) | 94 | 94 | 82 | 84 | 80 | 88 | 84 | 76 |
| QTc interval (ms) | 460 | 435 | 412 | 427 | 420 | 399 | 458 | 435 |
| CT aortic annulus area (cm2) | 4.8 | 3.8 | 6.1 | 4.1 | 4.9 | 3.5 | 3.59 | 4.3 |
| CT aortic annulus perimeter (mm) | 79.2 | 73.4 | 88.7 | 72.3 | 80.3 | 66.8 | 71.2 | 73.4 |
| Creatinine postprocedural peak (mg/dL) | 1.1 | 1.1 | 0.6 | 1.6 | 0.8 | 0.9 | 0.99 | 1.0 |
| Gender | Male | Male | Male | Male | Female | Female | Female | Female |
| Extracardiac arteriopathy | No | Yes | Yes | No | No | No | 0 | No |
| Non-insulin-dependent diabetes mellitus | No | No | No | No | No | No | 0 | No |
| New York Heart Association class | II | III | II | III | III | III | III | I |
| Baseline persistent/permanent AF | No | No | Yes | No | No | Yes | 0 | No |
| Postprocedural aortic regurgitation | No | No | Mild | Mild | None | Trace | Trace | No |
| Observed 1-year survival: alive (yes/no) | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Predicted 1-year survival: alive (yes/no/no prediction) | Yes | Yes | No prediction | Yes | Yes | No prediction | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollari, F.; Hitzl, W.; Rottmann, M.; Vogt, F.; Ledwon, M.; Langhammer, C.; Eckner, D.; Jessl, J.; Bertsch, T.; Pauschinger, M.; et al. A Machine Learning Model for the Accurate Prediction of 1-Year Survival in TAVI Patients: A Retrospective Observational Cohort Study. J. Clin. Med. 2023, 12, 5481. https://doi.org/10.3390/jcm12175481
Pollari F, Hitzl W, Rottmann M, Vogt F, Ledwon M, Langhammer C, Eckner D, Jessl J, Bertsch T, Pauschinger M, et al. A Machine Learning Model for the Accurate Prediction of 1-Year Survival in TAVI Patients: A Retrospective Observational Cohort Study. Journal of Clinical Medicine. 2023; 12(17):5481. https://doi.org/10.3390/jcm12175481
Chicago/Turabian StylePollari, Francesco, Wolfgang Hitzl, Magnus Rottmann, Ferdinand Vogt, Miroslaw Ledwon, Christian Langhammer, Dennis Eckner, Jürgen Jessl, Thomas Bertsch, Matthias Pauschinger, and et al. 2023. "A Machine Learning Model for the Accurate Prediction of 1-Year Survival in TAVI Patients: A Retrospective Observational Cohort Study" Journal of Clinical Medicine 12, no. 17: 5481. https://doi.org/10.3390/jcm12175481
APA StylePollari, F., Hitzl, W., Rottmann, M., Vogt, F., Ledwon, M., Langhammer, C., Eckner, D., Jessl, J., Bertsch, T., Pauschinger, M., & Fischlein, T. (2023). A Machine Learning Model for the Accurate Prediction of 1-Year Survival in TAVI Patients: A Retrospective Observational Cohort Study. Journal of Clinical Medicine, 12(17), 5481. https://doi.org/10.3390/jcm12175481