Lysinuric Protein Intolerance and Its Nutritional and Multisystemic Challenges in Pregnancy: A Case Report and Literature Review
Abstract
:1. Introduction
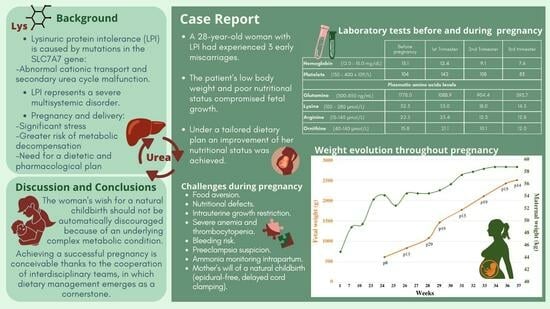
2. Case Report
2.1. Preconception Phase
2.2. Pregnancy
2.3. Delivery and Post-Partum
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauhin, W.; Habarou, F.; Gobin, S.; Servais, A.; Brassier, A.; Grisel, C.; Roda, C.; Pinto, G.; Moshous, D.; Ghalim, F.; et al. Update on Lysinuric Protein Intolerance, a Multi-faceted Disease Retrospective cohort analysis from birth to adulthood. Orphanet. J. Rare Dis. 2017, 12, 3. [Google Scholar] [CrossRef]
- Sebastio, G.; Sperandeo, M.P.; Andria, G. Lysinuric protein intolerance: Reviewing concepts on a multisystem disease. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157, 54–62. [Google Scholar] [CrossRef]
- Nunes, V.; Niinikoski, H. Lysinuric Protein Intolerance. In GeneReviews® [Internet]; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Tanner, L.M.; Näntö-Salonen, K.; Niinikoski, H.; Huoponen, K.; Simell, O. Long-term oral lysine supplementation in lysinuric protein intolerance. Metabolism 2007, 56, 185–189. [Google Scholar] [CrossRef]
- Tanner, L.; Näntö-Salonen, K.; Niinikoski, H.; Erkkola, R.; Huoponen, K.; Simell, O. Hazards associated with pregnancies and deliveries in lysinuric protein intolerance. Metabolism 2006, 55, 224–231. [Google Scholar] [CrossRef]
- Zhou, Y.; Dou, X.; Zhang, C.; He, R.; Ding, Y. Hyperammonemia in a pregnant woman with citrullinemia type I: A case report and literature review. BMC Pregnancy Childbirth 2022, 22, 950. [Google Scholar] [CrossRef]
- Wilcox, G. Impact of pregnancy on inborn errors of metabolism. Rev. Endocr. Metab. Disord. 2018, 19, 13–33. [Google Scholar] [CrossRef]
- Ünal, Ö.; Coşkun, T.; Orhan, D.; Tokatl, A.; Dursun, A.; Hişmi, B.; Özyüncü, Ö.; Sivri, S.H.K. Pregnancy and lactation outcomes in a Turkish patient with lysinuric protein intolerance. JIMD Rep. 2014, 13, 33–36. [Google Scholar] [CrossRef]
- Mikołajek-Bedner, W.; Torbé, A.; Kwiatkowski, S.; Michalczyk, M.; Gizewska, M.; Rokicki, D.; Rzepka, R.; Konstanty-Kurkiewicz, V.; Domański, M.; Czajka, R. Pregnancy delivery and puerperium in a patient with lysinuric protein intolerance—A case report. Ginekol. Pol. 2013, 84, 654–656. [Google Scholar] [CrossRef]
- Osada, H.; Seki, K. Amino acid changes during successful pregnancy in a case of lysinuric protein insufficiency. Gynecol. Obstet. Invest. 2006, 61, 139–141. [Google Scholar] [CrossRef]
- Manta-Vogli, P.D.; Schulpis, K.H.; Dotsikas, Y.; Loukas, Y.L. Nutrition and medical support during pregnancy and lactation in women with inborn errors of intermediary metabolism disorders (IEMDs). J. Pediatr. Endocrinol. Metab. 2020, 33, 5–20. [Google Scholar] [CrossRef]
- Walter, J.H. Inborn errors of metabolism and pregnancy. J. Inherit. Metab. Dis. 2000, 23, 229–236. [Google Scholar] [CrossRef]
- Stepien, K.M.; Geberhiwot, T.; Hendriksz, C.J.; Treacy, E.P. Challenges in diagnosing and managing adult patients with urea cycle disorders. J. Inherit. Metab. Dis. 2019, 42, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Hellmuth, C.; Uhl, O.; Buss, C.; Wadhwa, P.D.; Koletzko, B.; Entringer, S. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS ONE 2015, 10, e0145794. [Google Scholar] [CrossRef]
- Rama Rao, K.V.; Norenberg, M.D. Glutamine in the pathogenesis of hepatic encephalopathy: The Trojan horse hypothesis revisited. Neurochem. Res. 2014, 39, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Helling, G.; Wahlin, S.; Smedberg, M.; Pettersson, L.; Tjäder, I.; Norberg, Å.; Rooyackers, O.; Wernerman, J. Plasma glutamine concentrations in liver failure. PLoS ONE 2016, 11, e0150440. [Google Scholar] [CrossRef]
- De Las Heras, J.; Aldámiz-Echevarría, L.; Martínez-Chantar, M.-L.; Delgado, T.C. An update on the use of benzoate, phenylacetate and phenylbutyrate ammonia scavengers for interrogating and modifying liver nitrogen metabolism and its implications in urea cycle disorders and liver disease. Expert Opin. Drug Metab. Toxicol. 2017, 13, 439–448. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Navab, F. Lysinuric Protein Intolerance. Lancet 1980, 316, 703. [Google Scholar] [CrossRef]
- Kärki, M.; Näntö-Salonen, K.; Niinikoski, H.; Tanner, L.M. Urine Beta2-Microglobulin Is an Early Marker of Renal Involvement in LPI. JIMD Rep. 2016, 25, 47–55. [Google Scholar] [CrossRef]
- Fernández-Murray, J.P.; Prykhozhij, S.V.; Dufay, J.N.; Steele, S.L.; Gaston, D.; Nasrallah, G.K.; Coombs, A.J.; Liwski, R.S.; Fernandez, C.V.; Berman, J.N.; et al. Glycine and Folate Ameliorate Models of Congenital Sideroblastic Anemia. PLoS Genet. 2016, 12, e1005783. [Google Scholar] [CrossRef]
- Van Hove, J.L.K.; Kerckhove KVande Hennermann, J.B.; Mahieu, V.; Declercq, P.; Mertens, S.; De Becker, M.; Kishnani, P.S.; Jaeken, J. Benzoate treatment and the glycine index in nonketotic hyperglycinaemia. J. Inherit. Metab. Dis. 2005, 28, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Nikolac, N.; Omazic, J.; Simundic, A.M. The evidence-based practice for optimal sample quality for ammonia measurement. Clin. Biochem. 2014, 47, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Huizenga, J.R.; Tangerman, A.; Gips, C.H. Determination of ammonia in biological fluids. Ann. Clin. Biochem. 1994, 31, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Jóźwik, M.; Jóźwik, M.; Pietrzycki, B.; Chojnowski, M.; Teng, C.; Jóźwik, M.; Battaglia, F.C. Maternal and fetal blood ammonia concentrations in normal term human pregnancies. Biol. Neonate. 2005, 87, 38–43. [Google Scholar] [CrossRef] [PubMed]


| Reference Values | Before Pregnancy | 1st Trimester [1–13 Weeks] | 2nd Trimester [14–27 Weeks] | 3rd Trimester [28–37 Weeks] | 1–Year after Pregnancy | |
|---|---|---|---|---|---|---|
| Urea cycle evaluation | ||||||
| Ammonia (µmol/L) | 9–50 | 35 | 38 | 28 | 54↑ | 24 |
| Ur orotic acid (mmol/mol) | 0.4–5.1 | --- | --- | 3.9 | 2.7 | 1.4 |
| Plasma amino acids | ||||||
| Arg (µmol/L) | 15–140 | 22.5 | 25.4 | 12.3↓ | 12.8↓ | 19.9 |
| Cit (µmol/L) | 18–60 | 25.3 | 30.3 | 18.0 | 25.6 | 73.5 |
| Iso (µmol/L) | 40–100 | 55.4 | 48.6 | 23.7↓ | 24.0↓ | 43.2 |
| Leu (µmol/L) | 80–175 | 84.2 | 100.1 | 72.8↓ | 41.5↓ | 91.6 |
| Lys (µmol/L) | 100–280 | 32.3↓ | 53.0↓ | 18.0↓ | 14.3↓ | 53.7↓ |
| Met (µmol/L) | 20–40 | 32.4 | 27.1 | 14.9↓ | 17.8↓ | 25.9 |
| Gln (µmol/L) | 300–850 | 1778.0↑ | 1088.9↑ | 904.4↑ | 593.7 | 1052.6↑ |
| Glu (µmol/L) | 10–190 | 39.1 | 27.3 | 48.7 | 27.1 | 97.7 |
| Gly (µmol/L) | 130–350 | 520↑ | 421↑ | 280 | 229 | 424↑ |
| Orn (µmol/L) | 40–140 | 15.8↓ | 21.1↓ | 10.1↓ | 12.0↓ | 15.5↓ |
| Phe (µmol/L) | 30–100 | 32.4 | 37.7 | 30.0 | 23.1↓ | 35.6 |
| Thr (µmol/L) | 60–200 | 117.5 | 132.4 | 96.5 | 57.2 | 107.9 |
| Tyr (µmol/L) | 40–100 | 34.4↓ | 40.0 | 25.3↓ | 11.0↓ | 43.4 |
| Trp (µmol/L) | 30–120 | 23.3↓ | 21.0↓ | 16.6↓ | 10.7↓ | 22.8↓ |
| Val (µmol/L) | 140–340 | 226.3 | 174.1 | 118.1↓ | 94.9↓ | 180.7 |
| Nutritional status | ||||||
| Albumin (g/L) | 34–48 | 37 | 40 | 31↓ | 27↓ | 43 |
| Cholesterol (mg/dL) | <200 | 117 | 244↑ | 154 | 238↑ | 200 |
| Triglycerides (mg/dL) | <150 | 131 | 199 | 161 | 217↑ | 87 |
| Free carnitine (µmol/L) | 18.1–57.0 | 31.5 | 21.9 | 25.5 | 14.9↓ | 30.6 |
| Magnesium (mg/dL) | 1.8–2.6 | 1.3↓ | 1.8 | 1.4↓ | 1.3↓ | 1.4↓ |
| Calcium (mg/dL) | 8.5–10.5 | 8.6 | 9.2 | 8.3 | 8.2 | 8.8 |
| General renal function tests | ||||||
| Cr (mg/dL) | 0.3–1.3 | 0.45 | 0.58 | 0.35 | 0.44 | 0.44 |
| eGFR (CKD–EPI [Cr]) mL/min/1.73 m2 | ≥90 | 136 | 127 | 144 | 136 | 130 |
| Liver function tests | ||||||
| AST (U/L) | 5–40 | 48↑ | 67↑ | 53↑ | 107↑ | 121↑ |
| ALT (U/L) | 5–40 | 20 | 35 | 22 | 53↑ | 63↑ |
| ɣ–GT (U/L) | 5–40 | 7 | 18 | 14 | 34 | 16 |
| Before Pregnancy | 1st Trimester [1–13 Weeks] | 2nd Trimester [14–27 Weeks] | 3rd Trimester [28–37 Weeks] | |||
|---|---|---|---|---|---|---|
| Nutritional products | L-Citrulline (g/day) (g/intake) | Erratic consumption | 3 (1–1–1) | 6 (2–2–2) | 9 (3–3–3) | |
| Shake | Multifruit juice (mL/day) | --- | 1000 | 1000 | 1000 | |
| MCT oil (mL/day) (mL/intake) | --- | 15 (5–5–5) | 10 (5–0–5) | 10 (5–0–5) | ||
| EAA® (g/day) (g/intake) | --- | --- | 25 (8.3–8.3–8.3) | 25 (8.3–8.3–8.3) | ||
| Duocal® (g/day) (g/intake) | --- | 30 (10–10–10) | 30 (10–10–10) | --- | ||
| Vitajoule® (g/day) (g/intake) | --- | --- | --- | 60 (20–20–20) | ||
| Natural protein intake (g/day) * | 25 | 27 | 35 | 45–50 | ||
| Energy intake (Kcal/day) *Δ | 1000 | 1300 | 1550 | 1950 | ||
| Reference Values | Gestational Week 33 | Gestational Week 36 | 1–Year after Pregnancy | |
|---|---|---|---|---|
| Cr (mg/dL) | 0.3–1.3 | 0.44 | 0.43 | 0.44 |
| Cys C (mg/L) | 0.6–1.1 | 0.96 | 0.86 | 0.82 |
| eGFR (CKD–EPI [Cr]) | ≥90mL/min/1.73 m2 | 136 | 137 | 135 |
| eGFR (CKD–EPI [CysC]) | 87 | 101 | 107 | |
| Cr clearance (mL/min/1.73 m2) | 70–135 | 126 | 116 | 106 |
| Ur protein (mg/24 h) | <150 | 144 | 260↑ | 112 |
| Ur protein (mg/g Cr) | <200 | 250↑ | 726↑ | 104 |
| Reference Values | Before Pregnancy | 1st Trimester [1–13 Weeks] | 2nd Trimester [14–27 Weeks] | 3rd Trimester [28–37 Weeks] | 1–Year after Pregnancy | |
|---|---|---|---|---|---|---|
| Blood cells | ||||||
| RBC count × 1012/L | 3.80–4.8 | 4.69 | 4.43 | 2.55↓ | 3.52↓ | 4.94 |
| MCV (fl) | 80–100 | 90.4 | 87.9 | 90.2 | 91.0 | 91.4 |
| MCH (pg) | 26.7–33.0 | 27.9 | 28.1 | 29.4 | 29.7 | 28.4 |
| Reticulocytes × 109/L | 25.0–90.0 | 66.5 | – | 36.9 | 75.7 | 81.0 |
| Hemoglobin (mg/dL) | 12.0–15.0 | 13.1 | 12.4 | 9.1↓ | 7.6↓ | 14.0 |
| Hematocrit (L/L) | 0.36–0.46 | 0.42 | 0.40 | 0.24↓ | 0.31↓ | 0.45 |
| EPO (µmol/L) | 4.0–20.0 | – | – | – | 629.0 | 15.8 |
| WBC count × 109/L | 4–11 | 2.99↓ | 2.97↓ | 3.54↓ | 3.71↓ | 4.07 |
| Platelets (EDTA) × 109/L | 130–400 | 104↓ | 142 | 108↓ | 83↓ | 117↓ |
| Platelets (citrate) × 109/L | 150–400 | – | – | Platelets aggregates | – | Platelets aggregates |
| Micronutrients related to RBC function | ||||||
| Ferritin (ng/mL) | 15–200 | 474↑ | 756↑ | 1807↑ | 1017↑ | 1256↑ |
| RBC folate (ng/mL) | 250–1000 | 265 | 246↓ | 264 | 177↓ | 447 |
| Vit. B12 (pg/mL) | 250–1050 | 714 | 773 | 1084 | 795 | 1002 |
| Homocysteine (µmol/L) | <15 | – | – | – | 3.2 | 14.7 |
| Copper (µg/dL) | 70–140 | 39↓ | 61↓ | 131 | 138 | 66↓ |
| Selenium (µg/dL) | 60–150 | 43↓ | 47↓ | 58↓ | 50↓ | 62 |
| Zinc (µg/dL) | 59–110 | 140↑ | 225↑ | 84 | 55 | 107 |
| Hemolysis assessment | ||||||
| LDH (U/L) | <234 | 265↑ | 1740↑ | 1072↑ | 826↑ | 1144↑ |
| Total bilirubin (mg/dL) | <1.2 | 0.7 | 0.9 | 0.5 | 0.3 | 0.5 |
| Direct bilirubin (mg/dL) | <0.6 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 |
| Haptoglobin (g/L) | 0.32–1 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Coagulation tests | ||||||
| Prothrombin time (s) | 9.9–13.6 | 13.1 | 12.9 | 15.0 | 14.2 | 13.0 |
| Prothrombin time (%) | 80–100 | 86.2 | 86.3 | 68.1↓ | 68.4↓ | 92.7 |
| aPPT (s) | 23.5–32.0 | 24.6 | 24.4 | 26.3 | 29.7 | 25.6 |
| INR | 1.07 | 1.13 | 1.27 | 1.21 | 1.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pané, A.; Milad, C.; Santana-Domínguez, M.; Baños, N.; Borras-Novell, C.; Espinosa, G.; Magnano, L.; Nomdedeu, M.; Moreno-Lozano, P.J.; Cofan, F.; et al. Lysinuric Protein Intolerance and Its Nutritional and Multisystemic Challenges in Pregnancy: A Case Report and Literature Review. J. Clin. Med. 2023, 12, 6405. https://doi.org/10.3390/jcm12196405
Pané A, Milad C, Santana-Domínguez M, Baños N, Borras-Novell C, Espinosa G, Magnano L, Nomdedeu M, Moreno-Lozano PJ, Cofan F, et al. Lysinuric Protein Intolerance and Its Nutritional and Multisystemic Challenges in Pregnancy: A Case Report and Literature Review. Journal of Clinical Medicine. 2023; 12(19):6405. https://doi.org/10.3390/jcm12196405
Chicago/Turabian StylePané, Adriana, Camila Milad, Marta Santana-Domínguez, Núria Baños, Cristina Borras-Novell, Gerard Espinosa, Laura Magnano, Meritxell Nomdedeu, Pedro Juan Moreno-Lozano, Frederic Cofan, and et al. 2023. "Lysinuric Protein Intolerance and Its Nutritional and Multisystemic Challenges in Pregnancy: A Case Report and Literature Review" Journal of Clinical Medicine 12, no. 19: 6405. https://doi.org/10.3390/jcm12196405
APA StylePané, A., Milad, C., Santana-Domínguez, M., Baños, N., Borras-Novell, C., Espinosa, G., Magnano, L., Nomdedeu, M., Moreno-Lozano, P. J., Cofan, F., Placeres, M., Fernández, R. M., García-Villoria, J., Garrabou, G., Vinagre, I., Tanner, L. M., Montserrat-Carbonell, C., & Forga-Visa, M. d. T. (2023). Lysinuric Protein Intolerance and Its Nutritional and Multisystemic Challenges in Pregnancy: A Case Report and Literature Review. Journal of Clinical Medicine, 12(19), 6405. https://doi.org/10.3390/jcm12196405