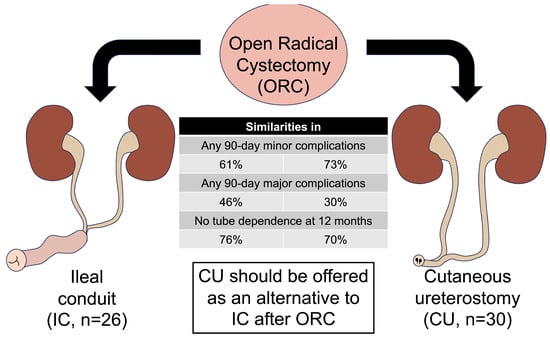
Ileal Conduit versus Cutaneous Ureterostomy after Open Radical Cystectomy: Comparison of 90-Day Morbidity and Tube Dependence at Intermediate Term Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Data Collection
2.3. Clinical Pathway
2.4. Follow-Up and Surveillance
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Operative Details and Hospital Stay
3.3. Perioperative Morbidity
3.4. Tube-Dependent Urinary Drainage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Max Bruins, H.; Carrion, A.; Cathomas, R.; Comperat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2024, 85, 17–31. [Google Scholar] [CrossRef]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Khetrapal, P.; Wong, J.K.L.; Tan, W.P.; Rupasinghe, T.; Tan, W.S.; Williams, S.B.; Boorjian, S.A.; Wijburg, C.; Parekh, D.J.; Wiklund, P.; et al. Robot-assisted Radical Cystectomy Versus Open Radical Cystectomy: A Systematic Review and Meta-analysis of Perioperative, Oncological, and Quality of Life Outcomes Using Randomized Controlled Trials. Eur. Urol. 2023, 84, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; McIntosh, A.G.; Strehlow, R.; Lawrence, V.A.; Parekh, D.J.; Svatek, R.S. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: A systematic review. Eur. Urol. 2013, 64, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Moon, A.; Thorpe, A.C. Metabolic complications of urinary intestinal diversion. Indian J. Urol. 2013, 29, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Novotny, V.; Zastrow, S.; Koch, R.; Wirth, M.P. Radical cystectomy in patients over 70 years of age: Impact of comorbidity on perioperative morbidity and mortality. World J. Urol. 2012, 30, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.F.; Burke, J.P.; McDermott, T.; Flynn, R.; Manecksha, R.P.; Thornhill, J.A. Bricker versus Wallace anastomosis: A meta-analysis of ureteroenteric stricture rates after ileal conduit urinary diversion. Can. Urol. Assoc. J. 2015, 9, E284–E290. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Naspro, R. Ileal Conduit as the Standard for Urinary Diversion After Radical Cystectomy for Bladder Cancer. Eur. Urol. Suppl. 2010, 9, 736–744. [Google Scholar] [CrossRef]
- Longo, N.; Imbimbo, C.; Fusco, F.; Ficarra, V.; Mangiapia, F.; Di Lorenzo, G.; Creta, M.; Imperatore, V.; Mirone, V. Complications and quality of life in elderly patients with several comorbidities undergoing cutaneous ureterostomy with single stoma or ileal conduit after radical cystectomy. BJU Int. 2016, 118, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Feminella, J.G., Jr.; Lattimer, J.K. A retrospective analysis of 70 cases of cutaneous ureterostomy. J. Urol. 1971, 106, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Keyes, E.L. Cutaneous Ureterostomy for the Relief of Intractable Bladder Tuberculosis. J. Urol. 1940, 44, 40–46. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; Lockhart, A.; King, J.; Wiegand, L.; Carrion, R.; Ordorica, R.; Lockhart, J. Cutaneous ureterostomy technique for adults and effects of ureteral stenting: An alternative to the ileal conduit. J. Urol. 2011, 186, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Lodde, M.; Pycha, A.; Palermo, S.; Comploj, E.; Hohenfellner, R. Uretero-ureterocutaneostomy (wrapped by omentum). BJU Int. 2005, 95, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, R.; Rodrigues Pessoa, R.; Dumbrava, M.G.; Packiam, V.T.; Thapa, P.; Tarrell, R.; Tollefson, M.K.; Jeffrey Karnes, R.; Frank, I.; Khanna, A.; et al. Cutaneous Ureterostomy Following Radical Cystectomy for Bladder Cancer: A Contemporary Series. Urology 2023, 181, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, F.C.; Wuethrich, P.Y. Cutaneous ureterostomy: ‘back to the future’. BJU Int. 2016, 118, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Korkes, F.; Fernandes, E.; Gushiken, F.A.; Glina, F.P.A.; Baccaglini, W.; Timoteo, F.; Glina, S. Bricker ileal conduit vs. Cutaneous ureterostomy after radical cystectomy for bladder cancer: A systematic review. Int. Braz. J. Urol. 2022, 48, 18–30. [Google Scholar] [CrossRef]
- Moeen, A.M.; Faragallah, M.A.; Zarzour, M.A.; Elbehairy, A.A.; Behnsawy, H.M. Ileal conduit versus single stoma uretero-cutanoustomy after radical cystectomy in patients >/= 75 years; which technique is better? a prospective randomized comparative study. Int. Urol. Nephrol. 2023, 55, 1719–1726. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Kavaric, P.; Eldin, S.; Nenad, R.; Dragan, P.; Vukovic, M. Modified wallace anastomotic technique reduces ureteroenteric stricture rates after ileal conduit urinary diversion. Int. Braz. J. Urol. 2020, 46, 446–455. [Google Scholar] [CrossRef]
- Thakker, P.U.; Refugia, J.M.; Casals, R.; Able, C.; Tsivian, M. Stent-free rates in cutaneous ureterostomy urinary diversion after radical cystectomy. Int. Urol. Nephrol. 2023, 55, 2809–2814. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Domenghino, A.; Walbert, C.; Birrer, D.L.; Puhan, M.A.; Clavien, P.A.; Outcome4Medicine consensus, g. Consensus recommendations on how to assess the quality of surgical interventions. Nat. Med. 2023, 29, 811–822. [Google Scholar] [CrossRef]
- Vetterlein, M.W.; Klemm, J.; Gild, P.; Bradtke, M.; Soave, A.; Dahlem, R.; Fisch, M.; Rink, M. Improving Estimates of Perioperative Morbidity After Radical Cystectomy Using the European Association of Urology Quality Criteria for Standardized Reporting and Introducing the Comprehensive Complication Index. Eur. Urol. 2020, 77, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Huber, T.; Pickl, C.; van Rhijn, B.W.G.; Guzvic, M.; Gierth, M.; Breyer, J.; Burger, M.; Mayr, R. The comprehensive complication index is associated with a significant increase in complication severity between 30 and 90 days after radical cystectomy for bladder cancer. Eur. J. Surg. Oncol. 2021, 47, 1163–1171. [Google Scholar] [CrossRef]
- Williams, S.B.; Cumberbatch, M.G.K.; Kamat, A.M.; Jubber, I.; Kerr, P.S.; McGrath, J.S.; Djaladat, H.; Collins, J.W.; Packiam, V.T.; Steinberg, G.D.; et al. Reporting Radical Cystectomy Outcomes Following Implementation of Enhanced Recovery After Surgery Protocols: A Systematic Review and Individual Patient Data Meta-analysis. Eur. Urol. 2020, 78, 719–730. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, R.; Liu, Z.; Qi, W.; Lv, G.; Zhong, M.; Liu, X.; Zhu, M.; Jiang, Z.; Chen, S.; et al. The effect of the enhanced recovery after surgery program on radical cystectomy: A meta-analysis and systematic review. Front. Surg. 2023, 10, 1101098. [Google Scholar] [CrossRef] [PubMed]
- Maibom, S.L.; Joensen, U.N.; Poulsen, A.M.; Kehlet, H.; Brasso, K.; Roder, M.A. Short-term morbidity and mortality following radical cystectomy: A systematic review. BMJ Open 2021, 11, e043266. [Google Scholar] [CrossRef]
- Ghoreifi, A.; Van Horn, C.M.; Xu, W.; Cai, J.; Miranda, G.; Bhanvadia, S.; Schuckman, A.K.; Daneshmand, S.; Djaladat, H. Urinary tract infections following radical cystectomy with enhanced recovery protocol: A prospective study. Urol. Oncol. 2020, 38, 75.e9–75.e14. [Google Scholar] [CrossRef] [PubMed]
- Beano, H.; He, J.; Hensel, C.; Worrilow, W.; Townsend, W.; Gaston, K.; Clark, P.E.; Riggs, S. Safety of decreasing ureteral stent duration following radical cystectomy. World J. Urol. 2021, 39, 473–479. [Google Scholar] [CrossRef]
- Donat, S.M.; Tan, K.S.; Jibara, G.; Dalbagni, G.; Carlon, V.A.; Sandhu, J. Intraoperative Ureteral Stent Use at Radical Cystectomy is Associated with Higher 30-Day Complication Rates. J. Urol. 2021, 205, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lannes, F.; Walz, J.; Maubon, T.; Rybikowski, S.; Fakhfakh, S.; Picini, M.; Tourret, M.; Brun, C.; Gravis, G.; Pignot, G. Enhanced Recovery after Surgery for Radical Cystectomy Decreases Postoperative Complications at Different Times. Urol. Int. 2022, 106, 171–179. [Google Scholar] [CrossRef] [PubMed]

| Variable | IC | CU | p-Value |
|---|---|---|---|
| n = 26 | n = 30 | ||
| Demographics | |||
| Age at RC, yr | 71 (62–76) | 74 (70–78) | 0.10 |
| Male sex | 22 (85) | 23 (77) | 0.52 |
| Caucasian race | 25 (96) | 26 (87) | 0.36 |
| ASA physical status > 3 | 4 (15) | 3 (10) | 0.69 |
| uCCI score | 2 (2–3) | 2 (2–3) | 0.31 |
| ECOG score > 1 | 1 (4) | 4 (13) | 0.36 |
| History of abdominal surgery | 8 (31) | 15 (50) | 0.18 |
| BMI ≥ 30 kg/m2 | 13 (50) | 3 (10) | 0.005 |
| Baseline GFR ≥ 60 mL/min per 1.73 m2 | 13 (50) | 9 (30) | 0.17 |
| Treatment details | |||
| MIBC on TURBT Pathology | 24 (92) | 24 (80) | 0.26 |
| Received neoadjuvant chemotherapy | 16 (61) | 15 (50) | 0.43 |
| ORC operating time, h | 4.9 (4.5–6.1) | 3.6 (3.1–4.2) | <0.0001 |
| Solitary kidney urinary diversion | 1 (4) | 1 (3) | >0.99 |
| Length of stay, d | 4 (4–6) | 3 (3–6) | 0.07 |
| Non-organ confined ORC pathology (pT3-pT4 or pN+) | 12 (46) | 14 (47) | >0.99 |
| 30-Day | 90-Day | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Complication | Management | IC n = 26 | CU n = 30 | p-Value | IC n = 26 | CU n = 30 | p-Value |
| Any (C.D. I to V) | 16 (61) | 20 (67) | >0.99 | 18 (69) | 23 (77) | 0.56 | ||
| CCI score | 21 (0–34) | 9 (0–31) | 0.80 | 21 (0–35) | 21 (6–41) | 0.87 | ||
| Minor (C.D. I to II) | 14 (54) | 20 (67) | 0.41 | 16 (61) | 22 (73) | 0.40 | ||
| C.D. I occurrences | ||||||||
| Paralytic ileus | Nasogastric tube | 1 (4) | 10 (33) | <0.01 | 2 (8) | 10 (33) | 0.02 | |
| C.D. II occurrences | ||||||||
| Anemia | Blood transfusion | 2 (8) | 3 (10) | >0.99 | 3 (11) | 3 (10) | >0.99 | |
| Pyelonephritis | Antibiotics | 3 (11) | 2 (7) | 0.65 | 5 (19) | 6 (20) | >0.99 | |
| C. difficile colitis | Antibiotics | 1 (4) | 1 (3) | >0.99 | 2 (8) | 1 (3) | 0.59 | |
| SSI—superficial | Antibiotics | 4 (15) | 1 (3) | 0.17 | 5 (19) | 3 (10) | 0.45 | |
| SSI—deep | Antibiotics | 4 (15) | 3 (10) | 0.69 | 6 (23) | 4 (13) | 0.49 | |
| Wound breakdown | Antibiotics | 2 (8) | 1 (3) | 0.59 | 2 (8) | 1 (3) | 0.59 | |
| Malnutrition | Nutritional support | 0 | 3 (10) | 0.24 | 1 (4) | 3 (10) | 0.61 | |
| Bacteremia | Antibiotics | 0 | 1 (3) | >0.99 | 1 (4) | 3 (10) | 0.61 | |
| Pneumonia | Antibiotics | 0 | 2 (7) | 0.49 | 0 | 2 (7) | 0.49 | |
| Atrial fibrillation | Chemical conversion | 1 (4) | 0 | 0.46 | 1 (4) | 0 | 0.46 | |
| Pulmonary embolism | Anticoagulation only | 0 | 1 (3) | >0.99 | 1 (4) | 1 (3) | >0.99 | |
| Major (C.D. III to V) | 8 (31) | 6 (20) | 0.38 | 12 (46) | 9 (30) | 0.27 | ||
| C.D. IIIa occurrences | ||||||||
| Ureteroenteric anastomotic leak | Ureteral stent | 2 (8) | - | - | 2 (8) | - | - | |
| Ureteral obstruction | Ureteral stent or nephrostomy tube placement | 0 | 3 (10) | 0.24 | 0 | 3 (10) | 0.24 | |
| Intra-abdominal abscess | Abdominal drain placement | 3 (11) | 0 | 0.09 | 3 (11) | 1 (3) | 0.32 | |
| Pelvic abscess | Pelvic drain placement | 2 (8) | 1 (3) | 0.59 | 2 (8) | 1 (3) | 0.59 | |
| Melena | Endoscopy | 0 | 1 (3) | >0.99 | 0 | 1 (3) | >0.99 | |
| Wound breakdown | Wound debridement | 0 | 0 | - | 1 (4) | 1 (3) | >0.99 | |
| C.D. IIIb occurrences | ||||||||
| Small bowel obstruction | Exploratory laparotomy, reduction in internal hernia | 0 | 0 | - | 1 (4) | 1 (3) | >0.99 | |
| Toxic megacolon | Exploratory laparotomy, bowel resection, fecal diversion | 0 | 0 | - | 1 (4) | 0 | 0.46 | |
| Failure to thrive | Gastrostomy tube placement | 0 | 0 | - | 1 (4) | 0 | 0.46 | |
| C.D. IVa occurrences | ||||||||
| Aspiration pneumonia | Intubation and mechanical ventilation | 0 | 1 (3) | >0.99 | 0 | 1 (3) | >0.99 | |
| Respiratory failure, COVID | ICU admission, respiratory support | 1 (4) | 0 | 0.46 | 1 (4) | 0 | 0.46 | |
| C.D. IVb occurrences | ||||||||
| Septic shock | ICU admission | 1 (4) | 3 (10) | 0.61 | 2 (8) | 3 (10) | >0.99 | |
| C.D. V occurrences | 0 | 0 | - | 0 | 1 (3) | >0.99 | ||
| Variable | IC | CU | p-Value |
|---|---|---|---|
| n = 26 | n = 22 | ||
| Post-op follow-up, mos | 16 (7–21) | 15 (10–20) | 0.92 |
| Drainage tube-dependent duration, d | 22 (16–32) | 75 (30–118) | <0.001 |
| Drainage tube-free duration, mos | 10 (4–18) | 10 (3–15) | 0.61 |
| Change in GFR from pre-op to most recent GFR a | 0 (−12–10) | −4.5 (−7.5–15) | 0.62 |
| Drainage tube replaced | 5 (19) | 7 (32) | 0.34 |
| Time to tube replacement, mos | 5 (2–6) | 3 (1–10) | 0.67 |
| Reason for replacement | |||
| Malignant external obstruction | 0 | 1 (5) | 0.46 |
| Abdominal wall stricture | - | 5 (23) | - |
| Uretero-enteric stricture | 3 (11) | - | - |
| Uretero-enteric leak | 1 (4) | - | - |
| Perirenal abscess | 1 (4) | 0 | >0.99 |
| Stomal stenosis | 0 | 1 (5) | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakker, P.U.; Refugia, J.M.; Wolff, D.; Casals, R.; Able, C.; Temple, D.; Rodríguez, A.R.; Tsivian, M. Ileal Conduit versus Cutaneous Ureterostomy after Open Radical Cystectomy: Comparison of 90-Day Morbidity and Tube Dependence at Intermediate Term Follow-Up. J. Clin. Med. 2024, 13, 911. https://doi.org/10.3390/jcm13030911
Thakker PU, Refugia JM, Wolff D, Casals R, Able C, Temple D, Rodríguez AR, Tsivian M. Ileal Conduit versus Cutaneous Ureterostomy after Open Radical Cystectomy: Comparison of 90-Day Morbidity and Tube Dependence at Intermediate Term Follow-Up. Journal of Clinical Medicine. 2024; 13(3):911. https://doi.org/10.3390/jcm13030911
Chicago/Turabian StyleThakker, Parth U., Justin Manuel Refugia, Dylan Wolff, Randy Casals, Corey Able, Davis Temple, Alejandro R. Rodríguez, and Matvey Tsivian. 2024. "Ileal Conduit versus Cutaneous Ureterostomy after Open Radical Cystectomy: Comparison of 90-Day Morbidity and Tube Dependence at Intermediate Term Follow-Up" Journal of Clinical Medicine 13, no. 3: 911. https://doi.org/10.3390/jcm13030911
APA StyleThakker, P. U., Refugia, J. M., Wolff, D., Casals, R., Able, C., Temple, D., Rodríguez, A. R., & Tsivian, M. (2024). Ileal Conduit versus Cutaneous Ureterostomy after Open Radical Cystectomy: Comparison of 90-Day Morbidity and Tube Dependence at Intermediate Term Follow-Up. Journal of Clinical Medicine, 13(3), 911. https://doi.org/10.3390/jcm13030911