Multimodality Imaging in Right Heart Tumors: Proposed Algorithm towards an Appropriate Diagnosis
Abstract
:1. Introduction
2. Imaging Modalities for Assessing Right Heart Tumors
- Identify, localize, and describe the tumor (including its origin, attachments, size, morphology, mobility, hemodynamic impact, tissue characteristics, and vascularity);
- Differentiate the tumor from other cardiac pathologies through a comprehensive differential diagnosis;
- Assess the consequences of the tumor;
- Evaluate whether the tumor is primary or secondary.
2.1. Chest Radiography
2.2. Echocardiography
- -
- Angiosarcomas and lymphomas are the most frequent types of primary malignant tumors of the right heart chambers;
- -
- Liver or ovarian cancers are the most frequent malignant tumors that metastasize to the right heart;
- -
- Angiosarcomas or lymphomas are more likely to infiltrate the atrial wall or extend to the pericardium;
- -
- If there is continuity with the inferior vena cava, consider assessing for liver or gynecological cancers; also, a thorough differential diagnosis with a thrombus or tumor thrombosis should be performed;
- -
- If there is continuity with the superior vena cava, consider lymphoma as a possible diagnosis;
- -
- In the presence of a single mass with a thin stalk, consider myxoma as a possible benign tumor; sessile masses are most likely infiltrative and malignant.
2.3. Cardiac Computer Tomography
- Size of the mass (assessed in terms of diameters or volumes);
- Tumor margins (circumscribed, micro-lobulated, obscured or partially hidden by adjacent tissue, indistinct or ill-defined, and spiculated);
- Invasiveness (disruption of neighboring tissue and extension of the mass into the tissue);
- Mass density, defined as solid or mainly cystic and rated as hypo-, iso-, or hyperdense (compared to the normal cardiac muscle);
- Presence of intramass calcifications;
- Contrast uptake, defined as an increase of at least 10 Hounsfield units in cardiac mass density compared to baseline;
- Pericardial effusion.
2.4. Cardiac Magnetic Resonance Imaging
2.5. Positron Emission Tomography-Computed Tomography
- Maximum standardized uptake value;
- Mean standardized uptake value;
- Metabolic tumor volume;
- Total lesion glycolysis.
2.6. Invasive Angiography
3. Imaging Features of Right Heart Tumors
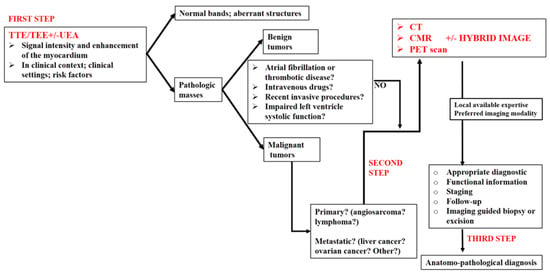
4. Proposed Algorithm for an Appropriate Multimodality Imaging Diagnosis in Right Heart Tumors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajiah, P.; MacNamara, J.; Chaturvedi, A.; Ashwath, R.; Fulton, N.L.; Goerne, H. Bands in the Heart: Multimodality Imaging Review. Radiographics 2019, 39, 1238–1263. [Google Scholar] [CrossRef]
- Han, B.K.; Rigsby, C.K.; Leipsic, J.; Bardo, D.; Abbara, S.; Ghoshhajra, B.; Lesser, J.R.; Raman, S.V.; Crean, A.M.; Nicol, E.D.; et al. Computed Tomography Imaging in Patients with Congenital Heart Disease, Part 2: Technical Recommendations. An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT): Endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2015, 9, 493–513. [Google Scholar]
- Secinaro, A.; Ait-Ali, L.; Curione, D.; Clemente, A.; Gaeta, A.; Giovagnoni, A.; Alaimo, A.; Esposito, A.; Tchana, B.; Sandrini, C.; et al. Recommendations for cardiovascular magnetic resonance and computed tomography in congenital heart disease: A consensus paper from the CMR/CCT working group of the Italian Society of Pediatric Cardiology (SICP) and the Italian College of Cardiac Radiology endorsed by the Italian Society of Medical and Interventional Radiology (SIRM) Part I. Radiol. Med. 2022, 127, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors: JACC Cardio Oncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tigadi, S.; Azrin, M.A.; Kim, A.S. Multimodality Imaging of a Right Atrial Cardiac Mass. Cureus 2019, 11, e4705. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, W.; Gao, L.; Ji, M.; Xie, M.; Li, Y. Multimodality Imaging of Benign Primary Cardiac Tumor. Diagnostics 2022, 12, 2543. [Google Scholar] [CrossRef]
- Șerban, A.; Dădârlat-Pop, A.; Tomoaia, R.; Trifan, C.; Molnar, A.; Manole, S.; Achim, A.; Suceveanu, M. The Role of Multimodality Imaging in the Diagnosis and Follow-Up of Malignant Primary Cardiac Tumors: Myxofibrosarcoma—A Case Report and Literature Review. Diagnostics 2023, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Pino, P.G.; Moreo, A.; Lestuzzi, C. Differential diagnosis of cardiac tumors: General consideration and echocardiographic approach. J. Clin. Ultrasound 2022, 50, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Quah, K.H.K.; Foo, J.S.; Koh, C.H. Approach to Cardiac Tumors Using Multimodal Cardiac Imaging. Curr. Probl. Cardiol. 2023, 48, 101731. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhao, R.; Yu, Y. Multimodality imaging in cardiac myxofibrosarcoma. Eur. Heart J. Case Rep. 2022, 6, ytac223. [Google Scholar] [CrossRef]
- Lanzoni, L.; Bonapace, S.; Dugo, C.; Chiampan, A.; Anselmi, A.; Ghiselli, L.; Molon, G. Cardiac Tumors and Contrast Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2022, 23, jeab289. [Google Scholar] [CrossRef]
- Chou, W.-H.; Chi, N.-H.; Wang, Y.-C.; Huang, C.-H. Metastatic breast cancer with right ventricular erosion. Eur. J. Cardio-Thoracic. Surg. 2016, 49, 1006–1007. [Google Scholar] [CrossRef] [PubMed]
- Bajdechi, M.; Onciul, S.; Costache, V.; Brici, S.; Gurghean, A. Right atrial lipoma: A case report and literature review. Exp. Ther. Med. 2022, 24, 697. [Google Scholar] [CrossRef]
- Floria, M.; Guedes, A.; Buche, M.; Deperon, R.; Marchandise, B. A rare primary cardiac tumour: Cavernous hemangioma of the tricuspid valve. Eur. J. Echocardiogr. J. Work. Group Echocardiogr. Eur. Soc. Cardiol. 2011, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Grecu, M.; Floria, M.; Tinică, G. Complication due to entrapment in the Chiari apparatus. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2014, 16, 577. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.C.; Paolisso, P.; Vitale, G.; Foà, A.; Bergamaschi, L.; Magnani, I.; Saturi, G.; Rinaldi, A.; Toniolo, S.; Renzulli, M.; et al. Diagnostic Accuracy of Cardiac Computed Tomography and 18-F Fluorodeoxyglucose Positron Emission Tomography in Cardiac Tumors. JACC Cardiovasc. Imaging 2020, 13, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Bijnens, J.; Bourgeois, T.; L’Hoyes, W.; Bogaert, J.; Rega, F. The elephant in the atrium: An unexpected diagnosis resulting in obstructive cardiogenic shock. Eur. Heart J. Cardiovasc. Imaging 2023, 25, e55. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ko, W.S.; Kim, S.J. Diagnostic test accuracies of F-18 FDG PET for characterisation of cardiac tumors compared to conventional imaging techniques: Systematic review and meta-analysis. Br. J. Radiol. 2022, 95, 20210263. [Google Scholar] [CrossRef]
- Shu, S.; Wang, J.; Zheng, C. From pathogenesis to treatment, a systemic review of cardiac lipoma. J. Cardiothorac. Surg. 2021, 16, 1. [Google Scholar] [CrossRef]
- Benz, D.C.; Fuchs, T.A.; Tanner, F.C.; Eriksson, U.; Yakupoglu, H.Y. Multimodality imaging of a right ventricular mass. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1184. [Google Scholar] [CrossRef]
- Polk, S.L.; Montilla-Soler, J.; Gage, K.L.; Parsee, A.; Jeong, D. Cardiac metastases in neuroendocrine tumors: 68: Ga-DOTATATE PET/CT with cardiac magnetic resonance correlation. Clin. Nucl. Med. 2020, 45, e201–e205. [Google Scholar] [CrossRef] [PubMed]
- Floria, M.; Gerard, M.; Louagie, Y.; Weynand, B.; Schroeder, E. Double papillary fibroelastoma: Beautiful, innocent flowers in the left heart. J. Clin. Ultrasound. 2014, 42, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Yuan, H.; Kong, X.; Wang, J.; Wang, J.; Zheng, C. The value of multimodality imaging in diagnosis and treatment of cardiac lipoma. BMC Med. Imaging 2021, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Al-Aidarous, S.; Khanji, M.Y. Advanced cavoatrial tumour thrombus as an unusual cause of right heart failure. Eur. Heart J. Cardiovasc. Imaging 2023, 25, e61. [Google Scholar] [CrossRef] [PubMed]
- Aghayev, A.; Cheezum, M.K.; Steigner, M.L.; Mousavi, N.; Padera, R.; Barac, A.; Kwong, R.Y.; Di Carli, M.F.; Blankstein, R. Multimodality imaging to distinguish between benign and malignant cardiac tumors. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2022, 29, 1504–1517. [Google Scholar]
- Lopez-Mattei, J.C.; Lu, Y. Multimodality Imaging in Cardiac Masses: To Standardize Recommendations, The Time Is Now! JACC Cardiovasc. Imaging 2020, 13, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Madan, N.; Lucas, J.; Akhter, N.; Collier, P.; Cheng, F.; Guha, A.; Zhang, L.; Sharma, A.; Hamid, A.; Ndiokho, I.; et al. Artificial intelligence and imaging: Opportunities in cardio-oncology. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 15, 100126. [Google Scholar] [CrossRef]
- Martinez, D.S.; Noseworthy, P.A.; Akbilgic, O.; Herrmann, J.; Ruddy, K.J.; Hamid, A.; Maddula, R.; Singh, A.; Davis, R.; Gunturkun, F.; et al. Artificial intelligence opportunities in cardio-oncology: Overview with spotlight on electrocardiography. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 15, 100129. [Google Scholar] [CrossRef]
- Baldassarre, L.A.; Ganatra, S.; Lopez-Mattei, J.; Yang, E.H.; Zaha, V.G.; Wong, T.C.; Ayoub, C.; DeCara, J.M.; Dent, S.; Deswal, A.; et al. Advances in Multimodality Imaging in Cardio-Oncology: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef]








| IMAGING MODALITY | Identify, Localize, and Characterize the Tumor | Aid in the Differential Diagnosis by Distinguishing Them from Other Cardiac Pathologies | Evaluate the Consequences in the Case of Pathologic Entities | Classify as Primary or Secondary Tumor |
|---|---|---|---|---|
| 2D/3D TTE/TEE (including UEAS) | ++ | ++ | ++ | + |
| CT (including contrast CT) | +++ | +++ | +++ | ++ |
| CMR | +++ | +++ | +++ | +++ |
| PET | ++ | ++ | + | ++++ |
| Invasive angiography | NA | NA | + | NA |
| Nuclear imaging | NA | NA | NA | +++ |
| Tumors. TUMORS TYPE AND CLINICAL PRESENTATIONS | ECHOCARDIOGRAPHY | COMPUTER TOMOGRAPHY | CARDIAC MAGNETIC RESONANCE | POSITRON EMISSION TOMOGRAPHY |
|---|---|---|---|---|
| MIXOMA: This is frequently diagnosed in middle-aged patients (30–60 years old). The typical triad of symptoms includes embolism, obstruction, and constitutional symptoms. | It is a mobile, round- or oval-shaped, heterogeneous echogenic mass attached to the endocardial surface, less commonly localized in the right atrium (especially in the left atrium, to the fossa ovalis). By contrast echocardiography, it appears as a partial or incomplete enhancement. | It appears as a heterogeneous, well-defined spherical or ovoid mass; a low-attenuation intracavitary mass with a lobular contour and calcification (which are more common in the right atrium); or as filling defects surrounded by enhanced intracardiac blood, hypo- or isoattenuating relative to the myocardium. Contrast-enhanced CT will show weak or absent enhancement. | Usually smooth, well-defined, lobular or oval mass; it appears heterogeneous or isointense on T1WI and heterogeneous or hyperintense on T2WI (because of the high extracellular water content). It is relatively hyperintense compared with the myocardium and hypointense relative to the blood pool. On resting: slight heterogeneous enhancement. On LGE images: patchy and more heterogeneous enhancement 10–15 min after gadolinium contrast administration. | It may manifest as a mildly hypermetabolic, hypodense area. |
| PAPILLARY FIBROELASTOMA: This can be an incidental finding or be associated with a cerebral or systemic embolic event. | It is small (usually less than 1.5 cm) and round; well-circumscribed; homogeneously textured appearance; a short pedicle; shimmering edges. It could be better visualized on 3D TEE, with echocardiography being the first step in the evaluation of embolic events. | It can be hard to see on moving valves. It appears as a focal low-attenuation mass with irregular borders on the valves’ surfaces. It can identify the anatomic location and attachment site, allowing simultaneous evaluation of the coronary arteries. | It can be hard to see on moving valves. It appears as a round, small, homogeneous mass attached to valvular leaflets. It exhibits isointense signal intensity relative to the myocardium on T1-weighted images and hypointense or hyperintense signal intensity on T2-weighted images. On cine CMR images, there is a hypointense signal intensity. There is usually no delayed gadolinium enhancement on LGE. | It is usually not necessary. |
| LIPOMA: This is frequently an incidental finding. It may be asymptomatic (even in large dimensions); fatigue; dyspnea on exertion; chest distress; palpitations; sudden death. | It identifies lipoma location and attachment, shape, and size. It appears to have hypoechogenic, homogenous echo intensity and well-defined borders. | Homogenous, encapsulated hypodense mass (between –45 HU and −100 HU). | It is essential in the differential diagnosis of liposarcomas. It has the same signal intensity as subcutaneous fat; hyperintense (T1W1 and T2W1); hypointense (in fat saturation sequences); T1/T2 value (ms): 255/65; post gadolinium: no enhancement. | It is usually not necessary, except in cases of suspected malignancy. |
| RHABDOMYOMA: This may be associated with symptoms of congestive heart failure, palpitations, or syncope. These symptoms may gradually disappear because of the spontaneous regression of the tumor. | It appears small, round, and lobulated. | Small, round, multiple homogenous hyperechoic masses of variable size, usually brighter than the surrounding myocardium; in contrast CT, it is hypodense. This allows differentiation from fibromas by deformation imaging. | On T1W1, rhabdomyomas appear isointense to slightly hyperintense (as on T2W1) compared with the surrounding myocardium. No delayed gadolinium enhancement on LGE. | It is usually not recommended, except in cases of suspected malignancy. |
| FIBROMA: This may be associated with fatal arrhythmias, heart failure, and sudden death. Surgical treatment is recommended, regardless of symptoms. | It appears as a large, intramural, well-delimited, non-contractile, solitary solid lesion within the myocardium, with central calcification. | It is described as an intramural, homogenous structure with sharp margins or infiltrative, with central calcification (a common feature of fibromas on CT), and soft-tissue attenuation, frequently without enhancement. | On T1WI, it is an isointense tumor, and on T2WI, it is a hypointense homogenous structure. On LGE, an intense delayed hyperenhancement is observed, without enhancement on resting. | It is usually not necessary, except in cases of suspected malignancy. |
| PARAGANGLIOMA: It is associated with symptoms such as tachycardia, tremors, palpitations, flushing, hypertension, or hypotension because of excessive secretion of catecholamines. | It appears as a granular, oval, heterogeneous structure, well-delimited, with a broad base. Sometimes, adjacent structures, like the superior vena cava, can be compressed. They are highly vascular structures. | It is a well-delimited heterogeneous structure with low attenuation. In contrast CT, it is characterized by marked heterogeneous enhancement. If the margins are poorly defined, invasion or extracardiac extension can be suspected. Coronary angiography is able to assess the relationship with the tumor. | It appears on T1WI as isointense or hypointense, and on T2WI as hyperintense, heterogeneous, and with peripheral rim enhancement. | It appears positive with an intense uptake of radiotracers. |
| HEMANGIOMA: It is an incidental finding; if symptomatic, the patient can present with chest pain, arrhythmias, heart failure, dyspnea on exertion, syncope, stroke, pericardial effusion, cardiac tamponade, and even sudden death. | It appears as a well-defined structure, endocardial or intramural, with oscillations during the cardiac cycle, good vascularization (it presents blood flow signals on color Doppler flow imaging), and obvious enhancement. | It is a well-defined structure with low or equal density, associated with heterogeneous intense enhancement and “vascular blush” on coronary angiography. | It appears on T1WI as heterogeneous isointense or hypointense, and on T2WI as hyperintense with heterogeneous enhancement. | It is usually not necessary, except in cases of suspected malignancy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floria, M.; Burlacu, A.; Morariu, P.C.; Oancea, A.-F.; Iov, D.-E.; Baroi, G.L.; Stafie, C.S.; Scripcariu, V.; Tănase, D.M. Multimodality Imaging in Right Heart Tumors: Proposed Algorithm towards an Appropriate Diagnosis. J. Clin. Med. 2024, 13, 1000. https://doi.org/10.3390/jcm13041000
Floria M, Burlacu A, Morariu PC, Oancea A-F, Iov D-E, Baroi GL, Stafie CS, Scripcariu V, Tănase DM. Multimodality Imaging in Right Heart Tumors: Proposed Algorithm towards an Appropriate Diagnosis. Journal of Clinical Medicine. 2024; 13(4):1000. https://doi.org/10.3390/jcm13041000
Chicago/Turabian StyleFloria, Mariana, Alexandru Burlacu, Paula Cristina Morariu, Alexandru-Florinel Oancea, Diana-Elena Iov, Genoveva Livia Baroi, Celina Silvia Stafie, Viorel Scripcariu, and Daniela Maria Tănase. 2024. "Multimodality Imaging in Right Heart Tumors: Proposed Algorithm towards an Appropriate Diagnosis" Journal of Clinical Medicine 13, no. 4: 1000. https://doi.org/10.3390/jcm13041000
APA StyleFloria, M., Burlacu, A., Morariu, P. C., Oancea, A. -F., Iov, D. -E., Baroi, G. L., Stafie, C. S., Scripcariu, V., & Tănase, D. M. (2024). Multimodality Imaging in Right Heart Tumors: Proposed Algorithm towards an Appropriate Diagnosis. Journal of Clinical Medicine, 13(4), 1000. https://doi.org/10.3390/jcm13041000