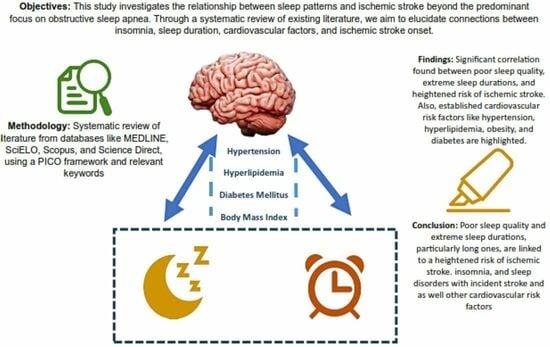
Exploring the Insomnia–Ischemic Stroke Nexus: A Comprehensive Review
Abstract
:1. Introduction
- Investigating the associations between insomnia and related sleep quality and ischemic stroke.
- Evaluating insomnia and sleep quality as a single risk factor for ischemic stroke, independently of or interacting with other cardiovascular risk factors (hypertension, obesity, hyperlipidemia or hypercholesterolemia, diabetes).
2. Method
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
- (a)
- Population
- (b)
- Outcomes
- (c)
- Secondary outcomes
- (d)
- Types of Studies
- (e)
- Due to the type of studies we intended to include, PICO intervention was also considered as variable of interest or exposure (i.e., exposure to the disease, risk behavior and/or prognostic factor). Comparison was also not chosen as an exclusion criteria.
2.3. Data Extraction
2.4. Study Quality
2.5. Data Analysis
3. Results
3.1. Study Characteristics
3.2. Demographic Characteristics
3.3. Study Setting
3.4. Insomnia and Insomnia-Related Symptoms Assessment
3.5. Stroke Assessment
3.6. Insomnia/Insomnia Symptoms Preceding Stroke
3.7. Impact of Insomnia/Insomnia Symptoms on Stroke Incidence
3.8. Stroke Characteristic
3.9. Association between Sleep Problems and Cardiovascular Risk Factors
Body Mass Index (BMI)
3.10. Hypertension
3.11. Diabetes Mellitus
3.12. Hyperlipidemia
3.13. Other Covariates
3.14. Mortality
3.15. Critical Appraisal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lau, H.L.; Rundek, T.; Ramos, A.R. Sleep and Stroke: New Updates on Epidemiology, Pathophysiology, Assessment, and Treatment. Curr. Sleep Med. Rep. 2019, 5, 71–82. [Google Scholar] [CrossRef]
- Koo, D.L.; Nam, H.; Thomas, R.J.; Yun, C.H. Sleep disturbances as a risk factor for stroke. J. Stroke 2018, 20, 12–32. [Google Scholar] [CrossRef]
- Brunetti, V.; Rollo, E.; Broccolini, A.; Frisullo, G.; Scala, I.; Della Marca, G. Sleep and Stroke: Opening Our Eyes to Current Knowledge of a Key Relationship. Curr. Neurol. Neurosci. Rep. 2022, 22, 767–779. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Personality Disorder, Severity Unspecified. In Encyclopedia of Personality and Individual Differences; Springer: Berlin/Heidelberg, Germany, 2020; p. 3798. [Google Scholar] [CrossRef]
- Gottlieb, E.; Landau, E.; Baxter, H.; Werden, E.; Howard, M.E.; Brodtmann, A. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Med. Rev. 2019, 45, 54–69. [Google Scholar] [CrossRef]
- Maniaci, A.; Riela, P.M.; Iannella, G.; Lechien, J.R.; La Mantia, I.; De Vincentiis, M.; Cammaroto, G.; Calvo-Henriquez, C.; Di Luca, M.; Estomba, C.C.; et al. Machine Learning Identification of Obstructive Sleep Apnea Severity through the Patient Clinical Features: A Retrospective Study. Life 2023, 13, 702. [Google Scholar] [CrossRef]
- Maniaci, A.; Ferlito, S.; Lechien, J.R.; Di Luca, M.; Iannella, G.; Cammaroto, G.; Cannavicci, A.; Pollicina, I.; Stilo, G.; Di Mauro, P.; et al. Anxiety, depression and sleepiness in OSA patients treated with barbed reposition pharyngoplasty: A prospective study. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4189–4198. [Google Scholar] [CrossRef]
- Hepburn, M.; Bollu, P.C.; French, B.; Sahota, P. Sleep Medicine Stroke and Sleep. Mo. Med. 2018, 115, 527–532. [Google Scholar] [PubMed]
- Mims, K.N.; Kirsch, D. Sleep and Stroke. Sleep Med. Clin. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Covassin, N.; Singh, P. Sleep Duration and Cardiovascular Disease Risk Epidemiologic and Experimental Evidence. Sleep Med. Clin. 2016, 11, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Pincherle, A.; Pace, M.; Sarasso, S.; Facchin, L.; Dreier, J.P.; Bassetti, C.L. Sleep, Preconditioning and Stroke. Stroke 2017, 48, 3400–3407. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Hoshide, S.; Ishikawa, S.; Shimada, K.; Kario, K. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. J. Am. Soc. Hypertens. 2010, 4, 255–262. [Google Scholar] [CrossRef]
- Ruiter, M.; Howard, V.J.; Letter, A.J.; Kleindorfer, D. Short sleep predicts stroke symptoms in persons of normal weight. Sleep 2012, 35, A279. [Google Scholar] [CrossRef]
- Amagai, Y.; Ishikawa, S.; Gotoh, T.; Kayaba, K.; Nakamura, Y.; Kajii, E. Sleep duration and incidence of cardiovascular events in a Japanese population: The Jichi medical school Cohort study. J. Epidemiol. 2010, 20, 106–110. [Google Scholar] [CrossRef]
- Petrov, M.E.; Howard, G.; Grandner, M.A.; Kleindorfer, D.; Molano, J.R.; Howard, V.J. Sleep duration and risk of incident stroke by age, sex, and race the REGARDS study. Neurology 2018, 91, E1702–E1709. [Google Scholar] [CrossRef] [PubMed]
- Helbig, A.K.; Stöckl, D.; Heier, M.; Ladwig, K.-H.; Meisinger, C. Symptoms of Insomnia and Sleep Duration and Their Association with Incident Strokes: Findings from the Population-Based MONICA/KORA Augsburg Cohort Study. PLoS ONE 2015, 10, e0134480. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.P.; Lin, H.J.; Weng, S.F.; Ho, C.H.; Wang, J.J.; Hsu, Y.W. Insomnia subtypes and the subsequent risks of stroke: Report from a nationally representative cohort. Stroke 2014, 45, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jin, X.; Shan, Z.; Li, S.; Huang, H.; Li, P.; Peng, X.; Peng, Z.; Yu, K.; Bao, W.; et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2017, 6, e005947. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Guo, X. Short and long sleep durations are both associated with increased risk of stroke: A meta-analysis of observational studies. Int. J. Stroke 2015, 10, 177–184. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chen, Y.-T.; Chen, M.-H.; Huang, C.-C.; Chiang, C.-H.; Huang, P.-H.; Chen, J.-W.; Chen, T.-J.; Lin, S.-J.; Leu, H.-B.; et al. The Association between Insomnia and Increased Future Cardiovascular Events: A Nationwide Population-Based Study. Psychosom. Med. 2015, 77, 743–751. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.; Brown, D.L.; Chervin, R.D. Sleep disorders and the risk of stroke. Expert. Rev. Neurother. 2018, 18, 523–531. [Google Scholar] [CrossRef]
- Sauvet, F.; Leftheriotis, G.; Gomez-Merino, D.; Langrume, C.; Drogou, C.; Van Beers, P.; Bourrilhon, C.; Florence, G.; Chennaoui, M. Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. 2010, 108, 68–75. [Google Scholar] [CrossRef]
- Carrington, M.J.; Trinder, J. Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep 2008, 31, 1701–1712. [Google Scholar] [CrossRef]
- Terzano, M.; Parrino, L.; Hala, T.E.R.; Bo, B. The nature of arousal in sleep. J. Sleep Res. 2004, 13, 1–23. [Google Scholar]
- Smolensky, M.H.; Hermida, R.C.; Portaluppi, F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med. Rev. 2017, 33, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Smolensky, M.H.; Fernández, J.R.; Mojón, A.; Portaluppi, F. Sleep-Time Blood Pressure: Unique Sensitive Prognostic Marker of Vascular Risk and Therapeutic Target for Prevention. Sleep Med. Rev. 2017, 33, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef] [PubMed]
- Tsiptsios, D.; Tsiptsios, D.; Leontidou, E.; Leontidou, E.; Fountoulakis, P.N.; Fountoulakis, P.N.; Ouranidis, A.; Ouranidis, A.; Matziridis, A.; Matziridis, A.; et al. Association between sleep insufficiency and dyslipidemia: A cross-sectional study among Greek adults in the primary care setting. Sleep Sci. 2022, 15, 49–58. [Google Scholar] [CrossRef]
- Chen, S.; Song, X.; Shi, H.; Li, J.; Ma, S.; Chen, L.; Lu, Y.; Hong, C.; Zhu, H.; Sun, H.; et al. Association Between Sleep Quality and Hypertension in Chinese Adults: A Cross-Sectional Analysis in the Tianning Cohort. Nat. Sci. Sleep 2022, 14, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, M. Associations. Aust. N. Z. J. Fam. Ther. 1992, 13, iii. [Google Scholar] [CrossRef]
- Caples, S.M. Sleep and Obesity. In Encyclopedia of Sleep; Academic Press: Cambridge, MA, USA, 2013; pp. 408–412. [Google Scholar] [CrossRef]
- Daniels, S.R. Sleep and obesity. J. Pediatr. 2018, 203, 3. [Google Scholar] [CrossRef]
- Spiegel, K.; Knutson, K.; Leproult, R.; Tasali, E.; Van Cauter, E. Sleep loss: A novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. 2005, 99, 2008–2019. [Google Scholar] [CrossRef]
- Thomas, S.J.; Calhoun, D. Sleep, insomnia, and hypertension: Current findings and future directions. J. Am. Soc. Hypertens. 2017, 11, 122–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Liu, J.; Lang, Y.; Liu, Y. The association between insomnia and the risk of metabolic syndrome: A systematic review and meta-analysis. J. Clin. Neurosci. 2021, 89, 430–436. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.W.; Hou, W.S.; Tang, Z.Y. Insomnia and risk of cardiovascular disease: A meta-analysis of cohort studies. Int. J. Cardiol. 2014, 176, 1044–1047. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Casini, A.; Macchi, C.; Abbate, R.; Gensini, G.F. Insomnia and risk of cardiovascular disease: A meta-analysis. Eur. J. Prev. Cardiol. 2014, 21, 57–64. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Song, M.; Wang, R.; Shi, L.; He, J.; Fan, T.-T.; Chen, W.-H.; Wang, L.; Yu, L.-L.; Gao, Y.-Y.; et al. Insomnia and multimorbidity in the community elderly in China. J. Clin. Sleep Med. 2017, 13, 591–597. [Google Scholar] [CrossRef]
- Canivet, C.; Nilsson, P.M.; Lindeberg, S.I.; Karasek, R.; Östergren, P.O. Insomnia increases risk for cardiovascular events in women and in men with low socioeconomic status: A longitudinal, register-based study. J. Psychosom. Res. 2014, 76, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, R.; Terecoasă, E.O.; Băjenaru, O.A.; Tiu, C. Etiologic classification of ischemic stroke: Where do we stand? Clin. Neurol. Neurosurg. 2017, 159, 93–106. [Google Scholar] [CrossRef]
- Cai, H.; Liang, J.; Liu, Z.; Fang, L.; Zheng, J.; Xu, J.; Chen, L.; Sun, W.; Zhang, H. Causal Effects of Sleep Traits on Ischemic Stroke and Its Subtypes: A Mendelian Randomization Study. Nat. Sci. Sleep 2020, 12, 783–790. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zheng, B.; Yu, C.; Lv, J.; Guo, Y.; Bian, Z.; Zhou, M.; Yang, L.; Chen, Y.; Li, X.; Zou, J.; et al. Insomnia symptoms and risk of cardiovascular diseases among 0.5 million adults: A 10-year cohort. Neurology 2019, 93, E2110–E2120. [Google Scholar] [CrossRef]
- Kim, J.H.; Hayek, S.S.; Ko, Y.-A.; Liu, C.; Tahhan, A.S.; Ali, S.; Alkhoder, A.; Gafeer, M.M.; Choudhary, F.; Bhimani, R.; et al. Sleep Duration and Mortality in Patients With Coronary Artery Disease. Am. J. Cardiol. 2019, 123, 874–881. [Google Scholar] [CrossRef]
- Pergola, B.L.; Moonie, S.; Pharr, J.; Bungum, T.; Anderson, J.L. Sleep duration associated with cardiovascular conditions among adult Nevadans. Sleep Med. 2017, 34, 209–216. [Google Scholar] [CrossRef]
- Chen, J.-C.; Brunner, R.L.; Ren, H.; Wassertheil-Smoller, S.; Larson, J.C.; Levine, D.W.; Allison, M.; Naughton, M.J.; Stefanick, M.L. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke 2008, 39, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, K.; Yang, L.; Wang, H.; Xiao, Y.; Qiu, G.; Liu, X.; Yuan, Y.; Bai, Y.; Li, X.; et al. Sleep duration, midday napping, and sleep quality and incident stroke: The Dongfeng-Tongji cohort. Neurology 2020, 94, e345–e356. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Park, S.H.; Kim, N.; Kim, W.; Park, J.H.; Ko, Y.; Yang, M.H.; Jang, M.S.; Han, M.; Jung, C.; et al. Trial of ORG 10172 in acute stroke treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. J. Am. Heart Assoc. 2014, 3, e001119. [Google Scholar] [CrossRef]
- Silva, L.A.S.; do Amaral, M.M.; Grassi, V.; Palmeira, A.L.R. Chronic insomnia disorder as risk factor for stroke: A systematic review. Arq. Neuropsiquiatr. 2022, 80, 1159–1166. [Google Scholar] [CrossRef]
- Wu, T.; Zou, Y.; Xu, K.; Jiang, X.; Zhou, M.; Zhang, S.; Song, C. Insomnia and multiple health outcomes: Umbrella review of meta-analyses of prospective cohort studies. Public Health 2023, 215, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lan, T.; Wang, Y.; Ren, L. Individual Insomnia Symptom and Increased Hazard Risk of Cardiocerebral Vascular Diseases: A Meta-Analysis. Front. Psychiatry 2021, 12, 654719. [Google Scholar] [CrossRef]
- Baylan, S.; Griffiths, S.; Grant, N.; Broomfield, N.M.; Evans, J.J.; Gardani, M. Incidence and Prevalence of Post-Stroke Insomnia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2020, 49, 101222. [Google Scholar] [CrossRef]

| Study | Country | Total N (Population) | Total N (%, n of Stroke) | Total N (%, n of Insomnia/Sleep Disturbance) | Design | Sampling | Mean Age, Years (SD) | Gender, % Male | Setting | Stroke Types | Follow Up, Years | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bang Zheng et al. (2019) [43] | China | 487,200 | 45,316 (9.3%) | 7431 (16.4%) insomnia symptoms | Prospective cohort study | Consecutive | 30 to 79 yo | 199,241 (40.9%) | China Kadoorie Biobank—recruited participants from 10 areas across China | NR | Median 9.6 y | NR |
| Jeong Hwan Kim et al. (2019) [44] | USA | 2846 | 68 (2%) | N = 1110 (39%) <6.5 h (short SD), N = 740 (26%), ≥6.5 h to <7.5 h (normal SD), and N = 996 (35%) ≥7.5 h (long SD) | Cross-sectional study | Consecutive | 64 yo ± 13 | 1775 (62.0%) | Emory Cardiovascular Biobank | NR | Median 2.8 y | 412 (15%) |
| Brianna L. Pergola et al. (2017) [45] | USA | 5101 | 218 (2.9%) | 61.8% slept 7–9 h on average, 31.5% slept 5–6 h, 3.8% slept 1–4 h, and 2.8% slept for 10–18 h | Cross-sectional, population-based study | Random digit dialed telephone survey | 18–34 yo: N = 895 (30.4%); 35–44 yo: N = 631 (17.6%); 45–64 yo: N = 1915 (33.9%); 65 yo: ˃65 yo N = 1607 (18.1%) | 2195 (50.2%) | 2013 Nevada Behavioral Risk Factor Surveillance System | NR | NA | NR |
| Jiu-Chiuan Chen et al. (2008) [46] | USA | 93,175 | 1166 (1.25%) | ≃8.3% short SD ≤ 5 h/night, 4.6% long sleepers (≥9 h/night) | Prospective study | Consecutive | 50–79 yo | 0% | Women’s Health Initiative Observational Study cohort | IS and non-IS | Average of 7.5 y | NR |
| Lue Zhou et al. (2020) [47] | China | 31,750 | 1151 definite IS; 287 probable IS (N = 1438 strokes, 4.5%) | 23.9% SD ≥ 9 h/night; 7.6% midday napping >90 min | Prospective cohort study | Consecutive | Average 61.7 yo | 13,996 (44.1%) | Dongfeng-Tongji cohort | IS, including subtypes * | Average 6.2 ± 2.4 y | NR |
| Study | Stroke Assessment | Insomnia/Other Sleep Disturbances Assessment | Subtype of Insomnia | Insomnia/Sleep Disturbance Overall Prevalence % | Development of Stroke Demographic Characteristics %, Mean (σ) | Other Sleep Characteristics Demographic Characteristics/Comorbidity |
|---|---|---|---|---|---|---|
| Bang Zheng et al. (2019) [43] | ICD-10 [stroke (I60–I61; I63–I64); IS (I63)] | Questionnaire—specific insomnia symptoms for at least 3 d/wk in the past month | DIMS, EMA, and DD | DIMS 55127 (11.3%); EMA 50691 (10.4%) | Associations of DD with the incidence of IS and its subtypes were Identified only in male participants, although the sex heterogeneity was not statistically significant | The associations of DIMS, EMA, and DD with total CVD incidence were consistently stronger in younger participants |
| Jeong Hwan Kim et al. (2019) [44] | Clinical diagnosis | Sleep questionnaire—“How many hours of sleep do you usually get each night (or when you usually sleep)?” | NA | NA | NR | Subjects reporting short SD tended to be younger, ♀, and black, with higher BMI, DM, and HF compared to those with normal SD. Those with long SD were older and more likely to have a history of hyperlipidemia, smoking, and prior MI. Independent predictors of short SD were ♀ sex, black race, and higher BMI, while older age and history of smoking were associated with long SD |
| Brianna L. Pergola et al. (2017) [45] | Clinical diagnosis | Standardized core questionnaire, along with optional modules, and state-added questions | NA | NA | After adjusting for age, sex, race, marital status, education, and insurance, ♂ were found to be 1.34 times more likely to report a IS compared to ♀, although this did not reach statistical significance. Individuals aged 18–34 yo were 95.8% less likely to report a IS compared to those aged 65 yo and older after adjusting for gender, age, race, marital status, education, and insurance. Those aged 35–44 yo and 45–64 yo were 78% and 59.1% less likely, respectively, than individuals 65 yo and older to have a IS (p-value < 0.05 for both) | Hypertensive individuals had a 3.67-fold higher likelihood of reporting stroke compared to those with normal blood pressure (p < 0.0001). High cholesterol was associated with a 2.32-fold increased likelihood of stroke (p < 0.0001). Diabetic individuals were nearly twice as likely to report a cardiovascular condition (p = 0.003), and those with depressive disorder had a 1.8-fold higher likelihood of a cardiovascular condition compared to those without (p < 0.05) |
| Jiu-Chiuan Chen et al. (2008) [46] | Clinical diagnosis | Questionnaire—“hours of sleep on a typical night during the past 4 weeks” (≤5, 6, 7, 8, 9, ≥10) | NA | Approximately 8.3% reported short SD ≤ 5 h/night, while 4.6% were long sleepers (≥9 h/night) | After adjusting for age and race, there was a 19% (95% CI: 3–37%) increase in RR in with ≤ 6 h/night of sleep. After further adjustment for socioeconomic status, depressive symptoms, HT use, and conventional CVD risk factors, the increased RR associated with ≤ 6 h/night of sleep became statistically non-significant. In contrast, there was a consistent and graded increase in RR observed for ♀ with 8 h/night of sleep and those with ≥9 h/night, with RRs increasing approximately by 25% among women with 8 h/night of sleep and by 70% in those with ≥9 h/night of sleep across all adjusted models. | Participants with higher BMI above the normal range were more likely to experience short DS, while current users of HT medication were less likely to report short SD. Those with existing coronary heart disease/cardiovascular disease, treated DM, hypertension, hypercholesterolemia, or depression had a higher likelihood of reporting short SD. ♀ with similar conditions were more likely to experience long SD. Individuals reporting longer SD or extended MN were more likely to be ♂, less educated, current smokers, current drinkers, and physically inactive. Longer SD was associated with a lower likelihood of hypertension, while extended MN was linked to a higher likelihood of hypertension, diabetes, and hyperlipidemia |
| Lue Zhou et al. (2020) [47] | ICD-10 codes I60–I61, I63–I64, I69.0–I69.1, and I69.3–I69.4 | Self-administrated questionnaire (SD, MN, and sleep quality) | NA | 23.9% slept ≥ 9 h/night and 7.6% reported MN > 90 min | The association of total stroke with long SD seemed to be more evident among individuals who were ≥65 yo, or with hypertension or hyperlipidemia or DM, but no interaction was observed except for DM (p for interaction = 0.033). Similarly, the risk of total stroke with long MN appeared to be more pronounced among persons who were overweight, but no interaction was found | Participants who reported SD ≥ 9 h/night or having MN ≥ 90 min were more likely to be ♂ less educated, current smokers, current drinkers, and physically inactive (all p < 0.05). Those reporting SD ≥ 9 h/night were less likely to have hypertension, whereas individuals having MN ≥ 90 min were more likely to have hypertension, DM, and hyperlipidemia compared to reference groups. |
| Screening Questions | Qualitative Studies | |||||||
|---|---|---|---|---|---|---|---|---|
| First Author | Year | Are There Clear Research Questions? | Does the Collected Data Allow Us to Address the Research Questions? | Is the Qualitative Approach Appropriate to Answer the Research Question? | Are the Qualitative Data Collection Methods Adequate to Address the Research Question? | Are the Findings Adequately Derived from the Data? | Is the Interpretation of Results Sufficiently Substantiated by Data? | Is There Coherence between Qualitative Data Sources, Collection, Analysis, and Interpretation? |
| Bang Zheng | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Jiu-Chiuan | 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Jeong Hwan Kim | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Brianna L. Pergola | 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lue Zhou | 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matas, A.; Pinto, N.; Conde, B.; Vaz Patto, M. Exploring the Insomnia–Ischemic Stroke Nexus: A Comprehensive Review. J. Clin. Med. 2024, 13, 1622. https://doi.org/10.3390/jcm13061622
Matas A, Pinto N, Conde B, Vaz Patto M. Exploring the Insomnia–Ischemic Stroke Nexus: A Comprehensive Review. Journal of Clinical Medicine. 2024; 13(6):1622. https://doi.org/10.3390/jcm13061622
Chicago/Turabian StyleMatas, Andreia, Nuno Pinto, Bebiana Conde, and Maria Vaz Patto. 2024. "Exploring the Insomnia–Ischemic Stroke Nexus: A Comprehensive Review" Journal of Clinical Medicine 13, no. 6: 1622. https://doi.org/10.3390/jcm13061622
APA StyleMatas, A., Pinto, N., Conde, B., & Vaz Patto, M. (2024). Exploring the Insomnia–Ischemic Stroke Nexus: A Comprehensive Review. Journal of Clinical Medicine, 13(6), 1622. https://doi.org/10.3390/jcm13061622