Upper-Limb Functional Recovery in Chronic Stroke Patients after COVID-19-Interrupted Rehabilitation: An Observational Study
Abstract
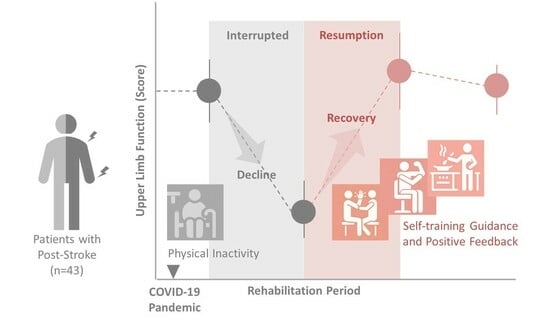
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Survey Periods and Instruments
2.4. Occupational Therapy for Outpatients
2.5. Main Outcome
2.6. Secondary Outcome
2.7. Participant Characteristics
2.8. Investigators
2.9. Statistical Analysis
2.10. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Examples of Self-Training Provided to Patients
| Exercise Items | Contents |
| Joint ROM exercises | Shoulder flexion, extension, abduction, adduction, internal rotation, and external rotation |
| Elbow flexion, extension, pronation, and supination | |
| Wrist flexion, extension. Radius flexion and ulnar flexion | |
| Finger flexion, extension, abduction, and adduction | |
| Stretching exercises | Pectoralis major muscle |
| Latissimus dorsi muscle | |
| Infraspinatus, supraspinatus, and teres minor | |
| Subscapularis muscle and teres major | |
| Biceps brachii muscle, brachii muscle, and brachioradialis | |
| Triceps brachii muscle | |
| Pronator quadratus and pronator teres | |
| Flexor carpi radialis and flexor carpi ulnaris | |
| Flexor digitourum profundus and flexor digitorum sperficials | |
| Adductor pollicis and opponens pollicis | |
| Lumbrical muscle, dorsal interossei, and palmar interossei | |
| ADL exercises | Holding a plate in place on a desk. |
| Holding in place a paper or a book on a desk. | |
| Smoothing creases in clothes. | |
| Grasping a plastic bottle with the paralyzed limb. | |
| Opening and closing a sliding door. | |
| Grasping a cell phone with the paralyzed hand. | |
| Manipulating a spoon or fork. | |
| Zipping and unzipping clothes; buttoning and unbuttoning clothes. | |
| Putting on socks. | |
| Tying shoelaces. | |
| Washing one’s face. | |
| Writing one’s signature. | |
| Drinking water from a cup. | |
| Drying laundry. | |
| Washing and tying hair. | |
| Manipulating chopsticks. | |
| Tying a necktie. | |
| Brushing teeth. | |
| Operating smartphones and PCs. | |
| Putting on and taking off necklaces and earrings. | |
| ADL, activities of daily living; ROM, range of motion. ADL exercises are performed by paralyzed arms and fingers. | |
Appendix B. Raw and Delta Scores of FMA-UE and ARAT during the Study Period, Shown by Sex
| Measurements | Date Period | Data | Female | Male | All | |
| FMA-UE | Total | 6 m before | Raw | 33.2 ± 14.0 | 33.5 ± 11.7 | 33.4 ± 12.5 |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 32.9 ± 14.3 | 33.4 ± 11.8 | 33.2 ± 12.7 | ||
| (1) | Delta | −0.2 ± 2.6 | −0.1 ± 2.5 | −0.1 ± 2.5 | ||
| Afterl IP | Raw | 31.8 ± 12.1 | 31.4 ± 10.7 | 31.6 ± 11.1 | ||
| (2) | Delta | −1.2 ± 3.6 | −2.0 ± 4.9 | −1.7 ± 4.4 | ||
| 3 m after | Raw | 32.4 ± 12.8 | 32.9 ± 11.5 | 32.7 ± 11.9 | ||
| (3) | Delta | 0.7 ± 3.3 | 1.4 ± 4.5 | 1.1 ± 4.1 | ||
| 6 m after | Raw | 33.1 ± 13.7 | 33.4 ± 11.1 | 33.3 ± 12.0 | ||
| (4) | Delta | 0.7 ± 3.3 | 0.5 ± 1.9 | 0.6 ± 2.5 | ||
| Part A | 6 m before | Raw | 24.3 ± 7.3 | 24.2 ± 5.8 | 24.2 ± 6.3 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 24.2 ± 7.7 | 24.3 ± 6.1 | 24.3 ± 6.7 | ||
| (1) | Delta | −0.1 ± 2.7 | 0.2 ± 1.4 | 0.1 ± 2.0 | ||
| Afterl IP | Raw | 23.6 ± 6.5 | 23.0 ± 5.7 | 23.3 ± 5.9 | ||
| (2) | Delta | −0.7 ± 2.5 | −1.3 ± 2.9 | −1.0 ± 2.7 | ||
| 3 m after | Raw | 24.0 ± 6.6 | 23.9 ± 5.4 | 24.0 ± 5.9 | ||
| (3) | Delta | 0.4 ± 1.3 | 0.9 ± 2.4 | 0.7 ± 2.0 | ||
| 6 m after | Raw | 24.3 ± 6.7 | 24.3 ± 5.5 | 24.3 ± 5.9 | ||
| (4) | Delta | 0.3 ± 1.8 | 0.4 ± 1.5 | 0.4 ± 1.6 | ||
| Part B | 6 m before | Raw | 3.2 ± 2.9 | 3.8 ± 2.8 | 3.5 ± 2.8 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 3.5 ± 2.9 | 3.7 ± 2.8 | 3.6 ± 2.8 | ||
| (1) | Delta | 0.3 ± 1.3 | 0.0 ± 0.9 | 0.1 ± 1.1 | ||
| Afterl IP | Raw | 3.5 ± 2.5 | 3.5 ± 2.8 | 3.5 ± 2.7 | ||
| (2) | Delta | 0.1 ± 1.4 | −0.3 ± 1.2 | −0.1 ± 1.3 | ||
| 3 m after | Raw | 3.5 ± 2.7 | 3.3 ± 2.8 | 3.4 ± 2.7 | ||
| (3) | Delta | 0.0 ± 1.4 | −0.2 ± 1.0 | −0.1 ± 1.2 | ||
| 6 m after | Raw | 3.8 ± 2.8 | 3.5 ± 2.5 | 3.6 ± 2.6 | ||
| (4) | Delta | 0.2 ± 0.9 | 0.2 ± 0.8 | 0.2 ± 0.8 | ||
| Part C | 6 m before | Raw | 4.1 ± 3.9 | 4.4 ± 3.2 | 4.3 ± 3.5 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 3.7 ± 3.7 | 4.2 ± 3.1 | 4.0 ± 3.3 | ||
| (1) | Delta | −0.4 ± 0.8 | −0.2 ± 1.2 | −0.3 ± 1.1 | ||
| Afterl IP | Raw | 3.6 ± 3.2 | 3.9 ± 2.9 | 3.8 ± 3.0 | ||
| (2) | Delta | −0.1 ± 1.2 | −0.4 ± 1.8 | −0.3 ± 1.6 | ||
| 3 m after | Raw | 3.5 ± 3.6 | 4.4 ± 3.5 | 4.0 ± 3.5 | ||
| (3) | Delta | −0.1 ± 1.2 | 0.5 ± 2.3 | 0.3 ± 1.9 | ||
| 6 m after | Raw | 3.8 ± 4.1 | 4.3 ± 3.2 | 4.1 ± 3.6 | ||
| (4) | Delta | 0.4 ± 1.0 | −0.1 ± 0.9 | 0.1 ± 1.0 | ||
| Part D | 6 m before | Raw | 1.6 ± 1.7 | 1.2 ± 1.8 | 1.3 ± 1.8 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 1.5 ± 1.7 | 1.2 ± 1.7 | 1.3 ± 1.7 | ||
| (1) | Delta | −0.1 ± 0.4 | 0.0 ± 0.6 | 0.0 ± 0.6 | ||
| Afterl IP | Raw | 1.1 ± 1.4 | 1.0 ± 1.5 | 1.0 ± 1.4 | ||
| (2) | Delta | −0.5 ± 1.4 | −0.1 ± 1.3 | −0.3 ± 1.4 | ||
| 3 m after | Raw | 1.4 ± 2.0 | 1.2 ± 1.7 | 1.3 ± 1.8 | ||
| (3) | Delta | 0.4 ± 0.7 | 0.2 ± 1.3 | 0.3 ± 1.1 | ||
| 6 m after | Raw | 1.2 ± 1.9 | 1.2 ± 1.8 | 1.2 ± 1.8 | ||
| (4) | Delta | −0.2 ± 1.0 | 0.0 ± 0.3 | −0.1 ± 0.7 | ||
| ARAT | Total | 6 m before | Raw | 10.8 ± 12.1 | 11.4 ± 12.7 | 11.1 ± 12.3 |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 9.7 ± 9.0 | 10.8 ± 12.2 | 10.4 ± 10.9 | ||
| (1) | Delta | −1.1 ± 5.2 | −0.5 ± 2.5 | −0.7 ± 3.8 | ||
| After IP | Raw | 7.8 ± 8.5 | 10.4 ± 12.6 | 9.4 ± 11.1 | ||
| (2) | Delta | −1.9 ± 3.4 | −0.4 ± 3.2 | −1.0 ± 3.3 | ||
| 3 m after | Raw | 9.9 ± 10.9 | 10.0 ± 12.3 | 10.0 ± 11.6 | ||
| (3) | Delta | 2.1 ± 3.4 | −0.4 ± 3.6 | 0.6 ± 3.7 | ||
| 6 m after | Raw | 9.8 ± 11.1 | 10.3 ± 12.4 | 10.1 ± 11.8 | ||
| (4) | Delta | −0.1 ± 0.4 | 0.3 ± 2.2 | 0.1 ± 1.7 | ||
| Grasp | 6 m before | Raw | 2.6 ± 4.2 | 3.1 ± 4.5 | 2.9 ± 4.4 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 2.3 ± 3.4 | 3.2 ± 4.5 | 2.8 ± 4.1 | ||
| (1) | Delta | −0.3 ± 1.4 | 0.0 ± 1.2 | −0.1 ± 1.3 | ||
| After IP | Raw | 1.6 ± 3.3 | 3.0 ± 4.9 | 2.4 ± 4.3 | ||
| (2) | Delta | −0.7 ± 2.0 | −0.2 ± 2.0 | −0.4 ± 2.0 | ||
| 3 m after | Raw | 2.2 ± 3.8 | 2.9 ± 4.6 | 2.6 ± 4.2 | ||
| (3) | Delta | 0.7 ± 1.6 | −0.1 ± 1.4 | 0.2 ± 1.5 | ||
| 6 m after | Raw | 2.3 ± 3.8 | 2.8 ± 4.6 | 2.6 ± 4.3 | ||
| (4) | Delta | 0.1 ± 0.2 | 0.0 ± 0.8 | 0.0 ± 0.7 | ||
| Grip | 6 m before | Raw | 2.0 ± 2.8 | 2.0 ± 2.9 | 2.0 ± 2.8 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 1.8 ± 2.2 | 2.1 ± 2.8 | 2.0 ± 2.6 | ||
| (1) | Delta | −0.2 ± 1.0 | 0.0 ± 0.7 | −0.1 ± 0.8 | ||
| After IP | Raw | 0.9 ± 1.7 | 2.0 ± 3.1 | 1.5 ± 2.7 | ||
| (2) | Delta | −0.9 ± 1.4 | −0.1 ± 1.4 | −0.4 ± 1.4 | ||
| 3 m after | Raw | 1.6 ± 2.6 | 1.8 ± 2.8 | 1.7 ± 2.7 | ||
| (3) | Delta | 0.7 ± 1.4 | −0.2 ± 1.1 | 0.2 ± 1.3 | ||
| 6 m after | Raw | 1.6 ± 2.6 | 1.8 ± 2.8 | 1.7 ± 2.7 | ||
| (4) | Delta | 0.0 ± 0.0 | 0.0 ± 0.7 | 0.0 ± 0.6 | ||
| Pinch | 6 m before | Raw | 1.8 ± 4.5 | 2.0 ± 4.2 | 1.9 ± 4.3 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 1.4 ± 3.2 | 1.6 ± 3.9 | 1.5 ± 3.6 | ||
| (1) | Delta | −0.4 ± 2.3 | −0.4 ± 1.4 | −0.4 ± 1.8 | ||
| After IP | Raw | 1.2 ± 3.2 | 1.6 ± 3.9 | 1.4 ± 3.6 | ||
| (2) | Delta | −0.2 ± 1.3 | 0.0 ± 0.5 | −0.1 ± 0.9 | ||
| 3 m after | Raw | 1.8 ± 4.2 | 1.4 ± 3.7 | 1.6 ± 3.8 | ||
| (3) | Delta | 0.6 ± 1.3 | −0.2 ± 0.8 | 0.1 ± 1.1 | ||
| 6 m after | Raw | 1.8 ± 4.0 | 1.5 ± 3.9 | 1.6 ± 3.9 | ||
| (4) | Delta | −0.1 ± 0.2 | 0.0 ± 0.2 | 0.0 ± 0.2 | ||
| Gross movement | 6 m before | Raw | 4.4 ± 1.6 | 4.2 ± 1.6 | 4.3 ± 1.5 | |
| - | Delta | - | - | - | ||
| 3 m before | Raw | 4.2 ± 1.3 | 4.0 ± 1.6 | 4.1 ± 1.4 | ||
| (1) | Delta | −0.2 ± 0.8 | −0.2 ± 0.5 | −0.2 ± 0.6 | ||
| After IP | Raw | 4.1 ± 1.2 | 3.9 ± 1.5 | 4.0 ± 1.4 | ||
| (2) | Delta | −0.1 ± 0.3 | −0.1 ± 0.7 | −0.1 ± 0.5 | ||
| 3 m after | Raw | 4.2 ± 1.4 | 4.3 ± 1.7 | 4.3 ± 1.6 | ||
| (3) | Delta | 0.1 ± 0.3 | 0.4 ± 0.8 | 0.3 ± 0.6 | ||
| 6 m after | Raw | 4.2 ± 1.4 | 4.2 ± 1.7 | 4.2 ± 1.6 | ||
| (4) | Delta | −0.1 ± 0.4 | 0.0 ± 0.2 | −0.1 ± 0.3 | ||
| Values are mean ± Std. deviation (n = 43). IP, interruption; FMA-UE, Fugl-Meyer assessment of the upper extremity; ARAT, Action Research Arm Test; 6 m before, approximately 6 months before outpatient rehabilitation was interrupted; 3 m before, approximately 3 months before outpatient rehabilitation was interrupted; after IP, after interruption period; 3 m after, approximately 3 months after outpatient rehabilitation was resumed; 6 m after, approximately 6 months after outpatient rehabilitation was resumed; (1) the amount of change in score from 6 months prior to interruption to 3 months prior to interruption; (2) the amount of change in scores from 3 months before interruption to immediately after resumption; (3) the amount of change in score from immediately after resumption to 3 months after resumption; (4) the amount of change in score from 3 months to 6 months after resumption. | ||||||
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 March 2023).
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the Effects of Non-Pharmaceutical Interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, S.; Allen, D.; Annan-Phan, S.; Bell, K.; Bolliger, I.; Chong, T.; Druckenmiller, H.; Huang, L.Y.; Hultgren, A.; Krasovich, E.; et al. The Effect of Large-Scale Anti-contagion Policies on the COVID-19 Pandemic. Nature 2020, 584, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Office for the Promotion of Countermeasures to Combat New Coronavirus Infections CS. Declaration of a State of Emergency in Response to the Novel Coronavirus Disease. Tokyo, Japan. 2020. Available online: http://japan.kantei.go.jp/ongoingtopics/_00018.html (accessed on 1 March 2023).
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Rodríguez-Sánchez, I.; Pérez-Rodríguez, P.; Ganz, F.; Torralba, R.; Oliveira, D.V.; Rodríguez-Mañas, L. Impact of Social Isolation due to COVID-19 on Health in Older People: Mental and Physical Effects and Recommendations. J. Nutr. Health Aging 2020, 24, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.; To, Q.G.; Khalesi, S.; Williams, S.L.; Alley, S.J.; Thwaite, T.L.; Fenning, A.S.; Vandelanotte, C. Depression, Anxiety and Stress during COVID-19: Associations with Changes in Physical Activity, Sleep, Tobacco and Alcohol Use in Australian Adults. Int. J. Environ. Res. Public Health 2020, 17, 4065. [Google Scholar] [CrossRef]
- van Mierlo, M.L.; van Heugten, C.M.; Post, M.W.; Hajós, T.R.; Kappelle, L.J.; Visser-Meily, J.M. Quality of Life during the First Two Years Post Stroke: The Restore4Stroke Cohort Study. Cerebrovasc. Dis. 2016, 41, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Palstam, A.; Sjödin, A.; Sunnerhagen, K.S. Participation and Autonomy Five Years after Stroke: A Longitudinal Observational Study. PLoS ONE 2019, 14, e0219513. [Google Scholar] [CrossRef]
- Legg, L.; Langhorne, P. Outpatient Service Trialists Rehabilitation Therapy Services for Stroke Patients Living at Home: Systematic Review of Randomised Trials. Lancet 2004, 363, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Assylbek, M.I.; Kocyigit, B.F.; Yessirkepov, M.; Zimba, O. Post-Stroke Rehabilitation in the Peri-Pandemic COVID-19 Era. Rheumatol. Int. 2024, 44, 399–411. [Google Scholar] [CrossRef]
- Zhu, C.; Tran, P.M.; Dreyer, R.P.; Goldstein, L.B.; Lichtman, J.H. Disparities in Internet Use among US Stroke Survivors: Implications for Telerehabilitation during COVID-19 and Beyond. Stroke 2022, 53, e90–e91. [Google Scholar] [CrossRef]
- Sakamoto, D.; Hamaguchi, T.; Nakayama, Y.; Hada, T.; Abo, M. Changes in Motor Paralysis Involving Upper Extremities of Outpatient Chronic Stroke Patients from Temporary Rehabilitation Interruption due to Spread of COVID-19 Infection: An Observational Study on Pre- and Post-Survey Data without a Control Group. PLoS ONE 2021, 16, e0260743. [Google Scholar] [CrossRef] [PubMed]
- Katrak, P.H.; Black, D.; Peeva, V. Do Stroke Patients with Intracerebral Hemorrhage Have a Better Functional Outcome than Patients with Cerebral Infarction? PM R 2009, 1, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Furie, K.L.; Shafqat, S.; Rallis, N.; Chang, Y.; Stein, J. Functional Recovery Following Rehabilitation after Hemorrhagic and Ischemic Stroke. Arch. Phys. Med. Rehabil. 2003, 84, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Maritz, R.; Aronsky, D.; Prodinger, B. The International Classification of Functioning, Disability and Health (ICF) in Electronic Health Records. A Systematic Literature Review. Appl. Clin. Inform. 2017, 8, 964–980. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The Post-Stroke Hemiplegic Patient. 1. A Method for Evaluation of Physical Performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, M.L.; Velozo, C.A.; Richards, L.G.; Duncan, P.W.; Studenski, S.; Lai, S.M. Dimensionality and Construct Validity of the Fugl-Meyer Assessment of the Upper Extremity. Arch. Phys. Med. Rehabil. 2007, 88, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Lyle, R.C. A Performance Test for Assessment of Upper Limb Function in Physical Rehabilitation Treatment and Research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Coupar, F.; Pollock, A.; Rowe, P.; Weir, C.; Langhorne, P. Predictors of Upper Limb Recovery after Stroke: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2012, 26, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, L.R.; Knutson, J.S.; Gunzler, D.; Chae, J. Relationship between Body Mass Index and Rehabilitation Outcomes in Chronic Stroke. Am. J. Phys. Med. Rehabil. 2012, 91, 951–956. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 Days of Bed Rest on Skeletal Muscle in Healthy Older Adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- English, C.; McLennan, H.; Thoirs, K.; Coates, A.; Bernhardt, J. Loss of Skeletal Muscle Mass After Stroke: A Systematic Review. Int. J. Stroke 2010, 5, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Sandek, A.; Doehner, W. Stroke-Related Sarcopenia: Specific Characteristics. J. Am. Med. Dir. Assoc. 2015, 16, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Hunnicutt, J.L.; Gregory, C.M. Skeletal Muscle Changes Following Stroke: A Systematic Review and Comparison to Healthy Individuals. Top. Stroke Rehabil. 2017, 24, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Allred, R.P.; Jefferson, S.C.; Kerr, A.L.; Woodie, D.A.; Cheng, S.Y.; Adkins, D.L. Motor System Plasticity in Stroke Models: Intrinsically Use-Dependent, Unreliably Useful. Stroke 2013, 44 (Suppl. S1), S104–S106. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J.; Milliken, G.W.; Jenkins, W.M.; Merzenich, M.M. Use-Dependent Alterations of Movement Representations in Primary Motor Cortex of Adult Squirrel Monkeys. J. Neurosci. 1996, 16, 785–807. [Google Scholar] [CrossRef]
- Classen, J.; Liepert, J.; Wise, S.P.; Hallett, M.; Cohen, L.G. Rapid Plasticity of Human Cortical Movement Representation Induced by Practice. J. Neurophysiol. 1998, 79, 1117–1123. [Google Scholar] [CrossRef]
- Peurala, S.H.; Kantanen, M.P.; Sjögren, T.; Paltamaa, J.; Karhula, M.; Heinonen, A. Effectiveness of Constraint-Induced Movement Therapy on Activity and Participation after Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Rehabil. 2012, 26, 209–223. [Google Scholar] [CrossRef]
- Kakuda, W.; Abo, M.; Sasanuma, J.; Shimizu, M.; Okamoto, T.; Kimura, C.; Kakita, K.; Hara, H. Combination Protocol of Low-Frequency rTMS and Intensive Occupational Therapy for Post-Stroke Upper Limb Hemiparesis: A 6-Year Experience of More than 1700 Japanese Patients. Transl. Stroke Res. 2016, 7, 172–179. [Google Scholar] [CrossRef]
- Winstein, C.; Kim, B.; Kim, S.; Martinez, C.; Schweighofer, N. Dosage Matters. Stroke 2019, 50, 1831–1837. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Plummer-D’Amato, P.; Elashoff, R.; Lee, J.; SIRROWS Group. International Randomized Clinical Trial, Stroke Inpatient Rehabilitation with Reinforcement of Walking Speed (SIRROWS), Improves Outcomes. Neurorehabil. Neural Repair 2010, 24, 235–242. [Google Scholar] [CrossRef]
- Stewart, J.C.; Lewthwaite, R.; Rocktashel, J.; Winstein, C.J. Self-Efficacy and Reach Performance in Individuals with Mild Motor Impairment Due to Stroke. Neurorehabil. Neural Repair 2019, 33, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Han, C.E.; Arbib, M.A.; Schweighofer, N. Stroke Rehabilitation Reaches a Threshold. PLoS Comput. Biol. 2008, 4, e1000133. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.B.; McIntyre, A.; Mirkowski, M.; Janzen, S.; Viana, R.; Britt, E.; Teasell, R. Patient-Centered Goal Setting in a Hospital-Based Outpatient Stroke Rehabilitation Center. PM R 2017, 9, 856–865. [Google Scholar] [CrossRef]
- Ekstrand, E.; Alt Murphy, M.; Persson, H.C.; Lundgren-Nilsson, Å.; Sunnerhagen, K.S. Which Clinical and Sociodemographic Determinants Are Associated with Self-Perceived Manual Ability at One Year after Stroke? Disabil. Rehabil. 2020, 42, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Landais, L.L.; Damman, O.C.; Schoonmade, L.J.; Timmermans, D.R.M.; Verhagen, E.A.L.M.; Jelsma, J.G.M. Choice Architecture Interventions to Change Physical Activity and Sedentary Behavior: A Systematic Review of Effects on Intention, Behavior and Health Outcomes during and after Intervention. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Germini, F.; Noronha, N.; Borg Debono, V.; Abraham Philip, B.; Pete, D.; Navarro, T.; Keepanasseril, A.; Parpia, S.; de Wit, K.; Iorio, A. Accuracy and Acceptability of Wrist-Wearable Activity-Tracking Devices: Systematic Review of the Literature. J. Med. Internet Res. 2022, 24, e30791. [Google Scholar] [CrossRef]
- Costantino, C.; Galuppo, L.; Romiti, D. Short-Term Effect of Local Muscle Vibration Treatment versus Sham Therapy on Upper Limb in Chronic Post-Stroke Patients: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 32–40. [Google Scholar] [CrossRef]
- Marcolino, M.A.Z.; Hauck, M.; Stein, C.; Schardong, J.; Pagnussat, A.S.; Plentz, R.D.M. Effects of Transcutaneous Electrical Nerve Stimulation Alone or as Additional Therapy on Chronic Post-Stroke Spasticity: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Disabil. Rehabil. 2020, 42, 623–635. [Google Scholar] [CrossRef]
- Widmer, M.; Held, J.P.; Wittmann, F.; Lambercy, O.; Lutz, K.; Luft, A.R. Does Motivation Matter in Upper-Limb Rehabilitation after Stroke? ArmeoSenso-Reward: Study Protocol for a Randomized Controlled Trial. Trials 2017, 18, 580. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Volpp, K.G.; Asch, D.A. Nudge Units to Improve the Delivery of Health Care. N. Engl. J. Med. 2018, 378, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, D.; Hamaguchi, T.; Murata, K.; Ito, H.; Nakayama, Y.; Abo, M. Upper Limb Function Recovery by Combined Repetitive Transcranial Magnetic Stimulation and Occupational Therapy in Patients with Chronic Stroke According to Paralysis Severity. Brain Sci. 2023, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, N.; Kawakami, M.; Ishii, R.; Tsuzuki, K.; Nakamura, T.; Okuyama, K.; Liu, M. Item Difficulty of Fugl-Meyer Assessment for Upper Extremity in Persons with Chronic Stroke with Moderate-to-Severe Upper Limb Impairment. Front. Neurol. 2020, 11, 577855. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, Y.; Kyougoku, M.; Takahashi, K.; Okita, Y.; Takebayashi, T. Dimensionality and Item-Difficulty Hierarchy of the Fugl-Meyer Assessment of the Upper Extremity Among Japanese Patients Who Have Experienced Stroke. Top. Stroke Rehabil. 2022, 29, 579–587. [Google Scholar] [CrossRef]
- Raghavan, P. Upper Limb Motor Impairment After Stroke. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Skinner, B.F. The Behavior of Organisms: An Experimental Analysis; BF Skinner Foundation: Cambridge, MA, USA, 2019. [Google Scholar]
- Santisteban, L.; Teremetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Aoike, F. Functional Recovery of Patients with Intracerebral Haemorrhage and Cerebral Infarction after Rehabilitation. Int. J. Rehabil. Res. 2021, 44, 222–225. [Google Scholar] [CrossRef]
- Tatsuno, H.; Hamaguchi, T.; Sasanuma, J.; Kakita, K.; Okamoto, T.; Shimizu, M.; Nakaya, N.; Abo, M. Does a Combination Treatment of Repetitive Transcranial Magnetic Stimulation and Occupational Therapy Improve Upper Limb Muscle Paralysis Equally in Patients with Chronic Stroke Caused by Cerebral Hemorrhage and Infarction?: A Retrospective Cohort Study. Medicine 2021, 100, e26339. [Google Scholar] [CrossRef]
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The Combined Influences of Exercise, Diet and Sleep on Neuroplasticity. Front. Psychol. 2022, 13, 831819. [Google Scholar] [CrossRef]
- Held, J.P.O.; Schwarz, A.; Pohl, J.; Thürlimann, E.; Porrtmann, S.; Branscheidt, M.; Fratian, M.; Van Duinen, J.; Veerbeek, J.M.; Luft, A.R. Changes in Stroke Rehabilitation during the SARS-CoV-2 Shutdown in Switzerland. J. Rehabil. Med. 2022, 54, jrm00272. [Google Scholar] [CrossRef] [PubMed]
- Schinwelski, M.; Sławek, J. Prevalence of Spasticity Following Stroke and Its Impact on Quality of Life with Emphasis on Disability in Activities of Daily Living. Systematic Review. Neurol. Neurochir. Pol. 2010, 44, 404–411. [Google Scholar] [CrossRef] [PubMed]




| No. | Contents |
|---|---|
| 1. Subjective symptoms of patients | |
| 1.1. | Interview the patient about subjective symptoms related to functional disability, activity limitations, and participation restrictions (Figure 2). Establish clear treatment goals based on the problems faced by the patient. |
| 1.2. | The therapist shares the treatment plan with the patient and provides practice and guidance. |
| 2. Upper-limb motor function | |
| 2.1. | The upper-limb functional assessment (FMA, ARAT) is performed. The joint range of motion and muscle tone are assessed. The acquired evaluation values are compared with those in the past, and changes in scores are checked. |
| 2.2. | Practice and instruction on upper extremity function with decreased ratings are provided. |
| 3. Use and practice of the upper limb on the paralyzed side | |
| 3.1. | The patient will be asked about ADL and practice using the paralyzed upper extremity. |
| 3.2. | The use of the paralyzed upper extremity for ADL will be promoted. The patient who lacked independent practice will be given feedback to improve their motivation. |
| Characteristics | Female | Male | All | |
|---|---|---|---|---|
| Participants | 17 (40) | 26 (60) | 43 (100) | |
| Age (years) | 50 [46, 63] | 53 [48, 60] | 51 [48, 60] | |
| Height (cm) | 158 [154, 164] | 170 [166, 173] | 166 [161, 171] | |
| Weight (kg) | 53 [51, 58] | 68 [62, 73] | 63 [53, 70] | |
| BMI (kg/m2) | 21 [20, 22] | 23 [23, 25] | 23 [21, 25] | |
| Diagnosis | CI | 8 (47) | 11 (42) | 19 (44) |
| ICH | 9 (53) | 15 (58) | 24 (56) | |
| Time from onset (months) | 139 [105, 172] | 122 [100, 165] | 133 [100, 167] | |
| Bartel Index | 100 [100] | 100 [100] | 100 [100] | |
| FMA-UE severity | Severe | 2 (12) | 4 (15) | 6 (14) |
| Moderate | 10 (59) | 18 (69) | 28 (65) | |
| Mild | 5 (29) | 4 (15) | 9 (21) | |
| Measurements | Raw | 6 m before | 3 m before | After IP | 3 m after | 6 m after | |
|---|---|---|---|---|---|---|---|
| Delta | - | (1) | (2) | (3) | (4) | ||
| FMA-UE | Total | Raw | 33.4 ± 12.5 | 33.2 ± 12.7 | 31.6 ± 11.1 | 32.7 ± 11.9 | 33.3 ± 12.0 |
| Delta | - | −0.1 ± 2.5 | −1.7 ± 4.4 | 1.1 ± 4.1 | 0.6 ± 2.5 | ||
| Part A | Raw | 24.2 ± 6.3 | 24.3 ± 6.7 | 23.3 ± 5.9 | 24.0 ± 5.9 | 24.3 ± 5.9 | |
| Delta | - | 0.1 ± 2.0 | −1.0 ± 2.7 | 0.7 ± 2.0 | 0.4 ± 1.6 | ||
| Part B | Raw | 3.5 ± 2.8 | 3.6 ± 2.8 | 3.5 ± 2.7 | 3.4 ± 2.7 | 3.6 ± 2.6 | |
| Delta | - | 0.1 ± 1.1 | −0.1 ± 1.3 | −0.1 ± 1.2 | 0.2 ± 0.8 | ||
| Part C | Raw | 4.3 ± 3.5 | 4.0 ± 3.3 | 3.8 ± 3.0 | 4.0 ± 3.5 | 4.1 ± 3.6 | |
| Delta | - | −0.3 ± 1.1 | −0.3 ± 1.6 | 0.3 ± 1.9 | 0.1 ± 1.0 | ||
| Part D | Raw | 1.3 ± 1.8 | 1.3 ± 1.7 | 1.0 ± 1.4 | 1.3 ± 1.8 | 1.2 ± 1.8 | |
| Delta | - | 0.0 ± 0.6 | −0.3 ± 1.4 | 0.3 ± 1.1 | −0.1 ± 0.7 | ||
| ARAT | Total | Raw | 11.1 ± 12.3 | 10.4 ± 10.9 | 9.4 ± 11.1 | 10.0 ± 11.6 | 10.1 ± 11.8 |
| Delta | - | −0.7 ± 3.8 | −1.0 ± 3.3 | 0.6 ± 3.7 | 0.1 ± 1.7 | ||
| Grasp | Raw | 2.9 ± 4.4 | 2.8 ± 4.1 | 2.4 ± 4.3 | 2.6 ± 4.2 | 2.6 ± 4.3 | |
| Delta | - | −0.1 ± 1.3 | −0.4 ± 2.0 | 0.2 ± 1.5 | 0.0 ± 0.7 | ||
| Grip | Raw | 2.0 ± 2.8 | 2.0 ± 2.6 | 1.5 ± 2.7 | 1.7 ± 2.7 | 1.7 ± 2.7 | |
| Delta | - | −0.1 ± 0.8 | −0.4 ± 1.4 | 0.2 ± 1.3 | 0.0 ± 0.6 | ||
| Pinch | Raw | 1.9 ± 4.3 | 1.5 ± 3.6 | 1.4 ± 3.6 | 1.6 ± 3.8 | 1.6 ± 3.9 | |
| Delta | - | −0.4 ± 1.8 | −0.1 ± 0.9 | 0.1 ± 1.1 | 0.0 ± 0.2 | ||
| Gross movement | Raw | 4.3 ± 1.5 | 4.1 ± 1.4 | 4.0 ± 1.4 | 4.3 ± 1.6 | 4.2 ± 1.6 | |
| Delta | - | −0.2 ± 0.6 | −0.1 ± 0.5 | 0.3 ± 0.6 | −0.1 ± 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, D.; Hamaguchi, T.; Nakayama, Y.; Hada, T.; Abo, M. Upper-Limb Functional Recovery in Chronic Stroke Patients after COVID-19-Interrupted Rehabilitation: An Observational Study. J. Clin. Med. 2024, 13, 2212. https://doi.org/10.3390/jcm13082212
Sakamoto D, Hamaguchi T, Nakayama Y, Hada T, Abo M. Upper-Limb Functional Recovery in Chronic Stroke Patients after COVID-19-Interrupted Rehabilitation: An Observational Study. Journal of Clinical Medicine. 2024; 13(8):2212. https://doi.org/10.3390/jcm13082212
Chicago/Turabian StyleSakamoto, Daigo, Toyohiro Hamaguchi, Yasuhide Nakayama, Takuya Hada, and Masahiro Abo. 2024. "Upper-Limb Functional Recovery in Chronic Stroke Patients after COVID-19-Interrupted Rehabilitation: An Observational Study" Journal of Clinical Medicine 13, no. 8: 2212. https://doi.org/10.3390/jcm13082212
APA StyleSakamoto, D., Hamaguchi, T., Nakayama, Y., Hada, T., & Abo, M. (2024). Upper-Limb Functional Recovery in Chronic Stroke Patients after COVID-19-Interrupted Rehabilitation: An Observational Study. Journal of Clinical Medicine, 13(8), 2212. https://doi.org/10.3390/jcm13082212