Reliability of Ultrasonographic Assessment of Depth of Invasion and Tumor Thickness in Intraoral Mucosa Lesions: A Preliminary Experience
Abstract
:1. Introduction
2. Methods
2.1. Patient Criteria
2.2. UHFUS Scan Protocol
2.3. Histopathological Assessment
2.4. Data Collection
2.5. Statistical Analysis
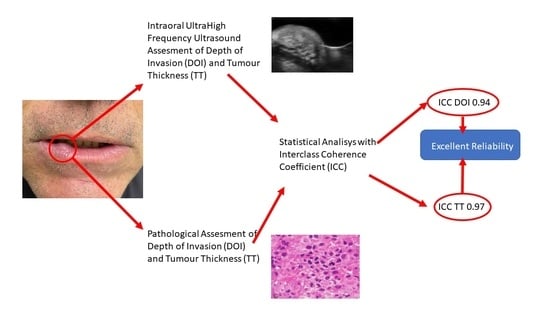
3. Results
3.1. Sample Characteristics
3.2. Interclass Coefficient Correlation
4. Discussion
- (a)
- Qualitative differences between probes: variations in probe performance and frequency emission can impact image quality and resolution, influencing the interpretation of ultrasound findings.
- (b)
- Intrinsic differences in anatomical areas: variations in tissue composition, thickness, and organization contribute to differences in ultrasound appearance between anatomical districts.
- (c)
- Intrinsic differences in tumor histology: variances in tumor histology across different neoplasms can lead to diverse ultrasound characteristics, complicating comparative analysis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Stasio, D.; Montella, M.; Romano, A.; Colella, G.; Serpico, R.; Lucchese, A. High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study. Cancers 2022, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.; Kukreja, P.; Mallick, I.; Roy, P. Worst pattern of invasion-type 4 (WPOI-4) and Lymphocyte host response should be mandatory reporting criteria for oral cavity squamous cell carcinoma: A re-look at the American Joint Committee of Cancer (AJCC) minimum dataset. Indian J. Pathol. Microbiol. 2020, 63, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Izzetti, R.; Nisi, M.; Aringhieri, G.; Vitali, S.; Oranges, T.; Romanelli, M.; Caramella, D.; Graziani, F.; Gabriele, M. Ultra-high frequency ultrasound in the differential diagnosis of oral pemphigus and pemphigoid: An explorative study. Ski. Res. Technol. 2021, 27, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.L.; Dedivitis, R.A.; Kulcsar, M.A.V.; de Mello, E.S.; Alves, V.A.F.; Cernea, C.R. External validation of the AJCC Cancer Staging Manual, 8th edition, in an independent cohort of oral cancer patients. Oral Oncol. 2017, 71, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.P. Eugene Nicholas Myers’ Lecture on Head and Neck Cancer, 2020: The Surgeon as a Prognostic Factor in Head and Neck Cancer Patients Undergoing Surgery. Int. Arch. Otorhinolaryngol. 2023, 27, e536–e546. [Google Scholar] [CrossRef]
- Gentile, E.; Maio, C.; Romano, A.; Laino, L.; Lucchese, A. The potential role of in vivo optical coherence tomography for evaluating oral soft tissue: A systematic review. J. Oral Pathol. Med. 2017, 46, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Di Stasio, D.; Petruzzi, M.; Serpico, R.; Lucchese, A. In vivo reflectance confocal microscopy of oral lichen planus. Int. J. Dermatol. 2019, 58, 940–945. [Google Scholar] [CrossRef]
- Kang, C.J.; Tsai, C.Y.; Lee, L.Y.; Lin, C.Y.; Yang, L.Y.; Cheng, N.M.; Hsueh, C.; Fan, K.H.; Wang, H.M.; Hsieh, C.H.; et al. Prognostic stratification of patients with AJCC 2018 pStage IVB oral cavity cancer: Should pT4b and pN3 disease be reclassified? Oral Oncol. 2021, 119, 105371. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.C.; Liao, C.T.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Kreppel, M.; Cernea, C.R.; et al. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: An international multicenter retrospective study. JAMA Otolaryngol.-Head Neck Surg. 2014, 140, 1138–1148. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Nardone, V.; D’Onofrio, I.; Salvia, A.A.H.; D’Ippolito, E.; Gallo, L.; Caliendo, V.; Gatta, G.; Fasano, M.; Grassi, R.; et al. Diffusion-weighted imaging and apparent diffusion coefficient mapping of head and neck lymph node metastasis: A systematic review. Explor. Target. Anti-Tumor Ther. 2022, 3, 734–745. [Google Scholar] [CrossRef]
- Harada, H.; Tomioka, H.; Hirai, H.; Kuroshima, T.; Oikawa, Y.; Nojima, H.; Sakamoto, J.; Kurabayashi, T.; Kayamori, K.; Ikeda, T. MRI before biopsy correlates with depth of invasion corrected for shrinkage rate of the histopathological specimen in tongue carcinoma. Sci. Rep. 2021, 11, 20992. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.; Filauro, M.; Iandelli, A.; Carobbio, A.L.C.; Mazzola, F.; Santori, G.; Parrinello, G.; Canevari, F.R.M.; Piazza, C.; Peretti, G. Magnetic Resonance vs. Intraoral Ultrasonography in the Preoperative Assessment of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.L.; Copel, J.A. Point-of-care ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Brown, J.; Rudralingam, M. The use of intraoral ultrasound in the characterization of minor salivary gland malignancy: Report of two cases. Dentomaxillofac. Radiol. 2016, 45, 20150354. [Google Scholar] [CrossRef] [PubMed]
- Angelelli, G.; Moschetta, M.; Limongelli, L.; Albergo, A.; Lacalendola, E.; Brindicci, F.; Favia, G.; Maiorano, E. Endocavitary sonography of early oral cavity malignant tumors. Head Neck 2017, 39, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Yoshihama, Y.; Ueyama, Y.; Terakado, N.; Kamei, S.; Fijimoto, Y.; Hasegawa, Y.; Matsuura, H.; Matsumura, T. The usefulness of intraoral ultrasonography in the evaluation of oral cancer. Int. J. Oral Maxillofac. Surg. 2001, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Chaudhari, A.J.; Sun, Y.; Zhou, F.; Dobbie, A.; Gandour-Edwards, R.F.; Tinling, S.P.; Farwell, D.G.; Monsky, W.L.; Shung, K.K.; et al. Ultrasound backscatter microscopy for imaging of oral carcinoma. J. Ultrasound Med. 2013, 32, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Belfiore, M.P.; Russo, A.; Turriziani, F.; Moscarella, E.; Troiani, T.; Brancaccio, G.; Ronchi, A.; Giunta, E.; Sica, A.; et al. A Preliminary Study for Quantitative Assessment with HFUS (High-Frequency Ultrasound) of Nodular Skin Melanoma Breslow Thickness in Adults Before Surgery: Interdisciplinary Team Experience. Curr. Radiopharm. 2020, 13, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Laverde-Saad, A.; Simard, A.; Nassim, D.; Jfri, A.; Alajmi, A.; O’Brien, E.; Wortsman, X. Performance of Ultrasound for Identifying Morphological Characteristics and Thickness of Cutaneous Basal Cell Carcinoma: A Systematic Review. Dermatology 2022, 238, 692–710. [Google Scholar] [CrossRef]
- Dinnes, J.; Bamber, J.; Chuchu, N.; Bayliss, S.E.; Takwoingi, Y.; Davenport, C.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; Deeks, J.J.; et al. High-frequency ultrasound for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, Cd013188. [Google Scholar] [CrossRef]
- Oranges, T.; Janowska, A.; Scatena, C.; Faita, F.; Lascio, N.D.; Izzetti, R.; Fidanzi, C.; Romanelli, M.; Dini, V. Ultra-High Frequency Ultrasound in Melanoma Management: A New Combined Ultrasonographic-Histopathological Approach. J. Ultrasound Med. 2023, 42, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Caramella, D.; Nisi, M.; Oranges, T.; Dini, V.; Graziani, F.; Gabriele, M. The efficacy of Ultra-High Frequency Ultrasonography in the diagnosis of intraoral lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 401–410. [Google Scholar] [CrossRef]
- Reginelli, A.; Russo, A.; Berritto, D.; Patane, V.; Cantisani, C.; Grassi, R. Ultra-High-Frequency Ultrasound: A Modern Diagnostic Technique for Studying Melanoma. Ultraschall Med. 2023, 44, 360–378. [Google Scholar] [CrossRef]
- Songra, A.K.; Ng, S.Y.; Farthing, P.; Hutchison, I.L.; Bradley, P.F. Observation of tumour thickness and resection margin at surgical excision of primary oral squamous cell carcinoma—Assessment by ultrasound. Int. J. Oral Maxillofac. Surg. 2006, 35, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Berritto, D.; Iacobellis, F.; Rossi, C.; Reginelli, A.; Cappabianca, S.; Grassi, R. Ultra high-frequency ultrasound: New capabilities for nail anatomy exploration. J. Dermatol. 2017, 44, 43–46. [Google Scholar] [CrossRef]
- McQueen, A.S.; Bhatia, K.S. Head and neck ultrasound: Technical advances, novel applications and the role of elastography. Clin. Radiol. 2018, 73, 81–93. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ Clin. Res. Ed. 2009, 339, b2700. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Reginelli, A.; Russo, A.; Russo, G.M.; Rocco, M.P.; Moscarella, E.; Ferrante, M.; Sica, A.; Grassi, R.; Cappabianca, S. Usefulness of High-Frequency Ultrasonography in the Diagnosis of Melanoma: Mini Review. Front. Oncol. 2021, 11, 673026. [Google Scholar] [CrossRef]
- Remonti, L.R.; Kramer, C.K.; Leitão, C.B.; Pinto, L.C.; Gross, J.L. Thyroid ultrasound features and risk of carcinoma: A systematic review and meta-analysis of observational studies. Thyroid Off. J. Am. Thyroid Assoc. 2015, 25, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Chang, J.L. Practical Salivary Ultrasound Imaging Tips and Pearls. Otolaryngol. Clin. N. Am. 2021, 54, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Tadayyon, H.; Sannachi, L.; Gangeh, M.; Sadeghi-Naini, A.; Tran, W.; Trudeau, M.E.; Pritchard, K.; Ghandi, S.; Verma, S.; Czarnota, G.J. Quantitative ultrasound assessment of breast tumor response to chemotherapy using a multi-parameter approach. Oncotarget 2016, 7, 45094–45111. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Marino, F.Z.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison with Computed Tomography-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Wortsman, X. Dermatology Ultrasound. Imaging Technique, Tips and Tricks, High-Resolution Anatomy. Ultrasound Q. 2020, 36, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Roldán, F.A.; Varelli, C.; Bard, R.; Corvino, A.; Wortsman, X. Skin cancer: Findings and role of high-resolution ultrasound. J. Ultrasound 2019, 22, 423–431. [Google Scholar] [CrossRef]
- Tarabichi, O.; Bulbul, M.G.; Kanumuri, V.V.; Faquin, W.C.; Juliano, A.F.; Cunnane, M.E.; Varvares, M.A. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: Systematic review. Laryngoscope 2019, 129, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.F.; Vartanian, J.G.; Pinto, C.A.L.; da Silva Rodrigues, I.F.P.; Kowalski, L.P. The impact of worst pattern of invasion on the extension of surgical margins in oral squamous cell carcinoma. Head Neck 2022, 44, 691–697. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Thankappan, K.; Battoo, A.J.; Rajapurkar, M.; Kuriakose, M.A.; Iyer, S. Isolated skip nodal metastasis is rare in T1 and T2 oral tongue squamous cell carcinoma. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2012, 147, 275–277. [Google Scholar] [CrossRef]
- Lee, M.K.; Choi, Y. Correlation between radiologic depth of invasion and pathologic depth of invasion in oral cavity squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2023, 136, 106249. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.C.; Liao, C.T.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Köhler, H.F.; Kreppel, M.; et al. Depth of invasion alone as an indication for postoperative radiotherapy in small oral squamous cell carcinomas: An International Collaborative Study. Head Neck 2019, 41, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.M.; Russo, A.; Urraro, F.; Cioce, F.; Gallo, L.; Belfiore, M.P.; Sangiovanni, A.; Napolitano, S.; Troiani, T.; Verolino, P.; et al. Management of Non-Melanoma Skin Cancer: Radiologists Challenging and Risk Assessment. Diagnostics 2023, 13, 793. [Google Scholar] [CrossRef] [PubMed]
- Chauvel-Picard, J.; Tognetti, L.; Cinotti, E.; Habougit, C.; Suppa, M.; Lenoir, C.; Rubegni, P.; Del Marmol, V.; Berot, V.; Gleizal, A.; et al. Role of ultra-high-frequency ultrasound in the diagnosis and management of basal cell carcinoma: Pilot study based on 117 cases. Clin. Exp. Dermatol. 2023, 48, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Dini, V.; Iannone, M.; Michelucci, A.; Manzo Margiotta, F.; Granieri, G.; Salvia, G.; Oranges, T.; Janowska, A.; Morganti, R.; Romanelli, M. Ultra-High Frequency UltraSound (UHFUS) Assessment of Barrier Function in Moderate-to-Severe Atopic Dermatitis during Dupilumab Treatment. Diagnostics 2023, 13, 2721. [Google Scholar] [CrossRef] [PubMed]
- Fulvio, G.; Izzetti, R.; Aringhieri, G.; Donati, V.; Ferro, F.; Gabbriellini, G.; Mosca, M.; Baldini, C. UHFUS: A Valuable Tool in Evaluating Exocrine Gland Abnormalities in Sjögren’s Disease. Diagnostics 2023, 13, 2771. [Google Scholar] [CrossRef]
- Taleb, E.; Yélamos, O.; Ardigo, M.; Christensen, R.E.; Geller, S. Non-invasive Skin Imaging in Cutaneous Lymphomas. Am. J. Clin. Dermatol. 2023, 25, 79–89. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Patanè, V.; Fusco, L.; Faggioni, L.; Boschetti, C.E.; Santagata, M.; Neri, E.; Cappabianca, S.; Reginelli, A. Reliability of Ultrasonographic Assessment of Depth of Invasion and Tumor Thickness in Intraoral Mucosa Lesions: A Preliminary Experience. J. Clin. Med. 2024, 13, 2595. https://doi.org/10.3390/jcm13092595
Russo A, Patanè V, Fusco L, Faggioni L, Boschetti CE, Santagata M, Neri E, Cappabianca S, Reginelli A. Reliability of Ultrasonographic Assessment of Depth of Invasion and Tumor Thickness in Intraoral Mucosa Lesions: A Preliminary Experience. Journal of Clinical Medicine. 2024; 13(9):2595. https://doi.org/10.3390/jcm13092595
Chicago/Turabian StyleRusso, Anna, Vittorio Patanè, Luigia Fusco, Lorenzo Faggioni, Ciro Emiliano Boschetti, Mario Santagata, Emanuele Neri, Salvatore Cappabianca, and Alfonso Reginelli. 2024. "Reliability of Ultrasonographic Assessment of Depth of Invasion and Tumor Thickness in Intraoral Mucosa Lesions: A Preliminary Experience" Journal of Clinical Medicine 13, no. 9: 2595. https://doi.org/10.3390/jcm13092595
APA StyleRusso, A., Patanè, V., Fusco, L., Faggioni, L., Boschetti, C. E., Santagata, M., Neri, E., Cappabianca, S., & Reginelli, A. (2024). Reliability of Ultrasonographic Assessment of Depth of Invasion and Tumor Thickness in Intraoral Mucosa Lesions: A Preliminary Experience. Journal of Clinical Medicine, 13(9), 2595. https://doi.org/10.3390/jcm13092595