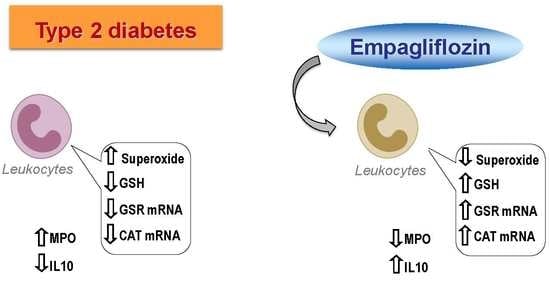
The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients and Sample Collection
2.2. Anthropometric and Biochemical Analyses
2.3. Leukocyte Extraction
2.4. Superoxide Production and GSH Content
2.5. Quantitative Analysis of GSR and CAT Gene Expression
2.6. Myeloperoxidase (MPO) and Interleukin 10 (IL-10) Levels
2.7. Statistical Analysis
3. Results
3.1. Anthropometric and Biochemical Parameters
3.2. Mitochondrial Superoxide Production and GSH Content in Leukocytes
3.3. Antioxidant Gene Expression
3.4. Inflammatory Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lahnwong, S.; Chattipakorn, S.C.; Chattipakorn, N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Curr. Diabetol. 2018, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Executive summary: Heart disease and stroke statistics—2016 update: A report from the American heart association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Mijares, A.; Rocha, M.; Rovira-Llopis, S.; Bañuls, C.; Bellod, L.; De Pablo, C.; Alvarez, A.; Roldan-Torres, I.; Sola-Izquierdo, E.; Victor, V.M. Human leukocyte/endothelial cell interactions and mitochondrial dysfunction in type 2 diabetic patients and their association with silent myocardial ischemia. Diabetes Care 2013, 36, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- DeFronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Anders, H.J.; Davis, J.M.; Thurau, K. Nephron protection in diabetic kidney disease. N. Engl. J. Med. 2016, 375, 2096–2098. [Google Scholar] [CrossRef]
- Wright, E.M. Renal Na(+)-glucose cotransporters. Am. J. Physiol. Renal Physiol. 2001, 280, F10–F18. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Norton, L.; Defronzo, R.A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 2011, 32, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Merovci, A.; Solis-Herrera, C.; Daniele, G.; Eldor, R.; Fiorentino, T.V.; Tripathy, D.; Xiong, J.; Perez, Z.; Norton, L.; Abdul-Ghani, M.A.; et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Investig. 2014, 124, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Gmyr, V.; Queniat, G.; Moerman, E.; Thévenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 2015, 21, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, G.; Hach, T.; Crowe, S.; Sanghvi, A.; Hall, K.D.; Ferrannini, E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015, 38, 1730–1735. [Google Scholar] [CrossRef]
- Rajasekeran, H.; Lytvyn, Y.; Cherney, D.Z.I. Sodium–glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: The emerging role of natriuresis. Kidney Int. 2016, 89, 524–526. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Zinman, B. Empagliflozin and progression of kidney disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1801–1802. [Google Scholar] [CrossRef]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Kohler, S.; Zeller, C.; Iliev, H.; Kaspers, S. Safety and Tolerability of Empagliflozin in Patients with Type 2 Diabetes: Pooled Analysis of Phase I-III Clinical Trials. Adv. Ther. 2017, 34, 1707–1726. [Google Scholar] [CrossRef]
- Sattar, N.; Petrie, M.C.; Zinman, B.; Januzzi, J.L., Jr. Novel diabetes drugs and the cardiovascular specialist. J. Am. Coll. Cardiol. 2017, 69, 2646–2656. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Dicembrini, I.; Kundisova, L.; Zannoni, S.; Nreu, B.; Mannucci, E. A meta-analysis of the hypoglycaemic risk in randomized controlled trials with sulphonylureas in patients with type 2 diabetes. Diabetes Obes. Metab. 2014, 16, 833–840. [Google Scholar] [CrossRef]
- Häring, H.U.; Merker, L.; Seewaldt-Becker, E.; Weime, M.; Meinicke, T.; Broedl, U.C.; Woerle, H.J. EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014, 37, 1650–1659. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, F.M.; Abraira, C.; Anderson, R.J.; Byington, R.P.; Chalmers, J.P.; Duckworth, W.C.; Evans, G.W.; Gerstein, H.C.; Holman, R.R.; Moritz, T.E.; et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009, 52, 2288–2298. [Google Scholar] [CrossRef]
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA 2015, 313, 603–615. [Google Scholar] [CrossRef]
- Dalama, B.; Mesa, J. New oral hypoglycemic agents and cardiovascular risk. Crossing the metabolic border. Rev. Esp. Cardiol. 2016, 69, 1088–1097. [Google Scholar] [CrossRef]
- Bosch, A.; Ott, C.; Jung, S.; Striepe, K.; Karg, M.V.; Kannenkeril, D.; Dienemann, T.; Schmieder, R.E. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc. Diabetol. 2019, 18, 44. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium-glucose cotransporter inhibitors and oxidative stress: An update. J. Cell Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef]
- Oelze, M.; Kröller-Schön, S.; Welschof, P.; Jansen, T.; Hausding, M.; Mikhed, Y.; Stamm, P.; Mader, M.; Zinßius, E.; Agdauletova, S.; et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE 2014, 9, e112394. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Chung, S.; Kim, S.J.; Lee, E.M.; Yoo, Y.H.; Kim, J.W.; Ahn, Y.B.; Kim, E.S.; Moon, S.D.; Kim, M.J.; et al. Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS ONE 2016, 11, e0165703. [Google Scholar] [CrossRef] [PubMed]
- Osorio, H.; Coronel, I.; Arellano, A.; Pacheco, U.; Bautista, R.; Franco, M.; Escalante, B. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid. Med. Cell Longev. 2012, 2012, 542042. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, T.; Zhu, S.; Guo, G.; Matsumoto, A.; Zhao, J.; Endo, M.; Horiguchi, H.; Morinaga, J.; Tian, Z.; Kadomatsu, T.; et al. Treatment of diabetic mice with the SGLT2 inhibitor TA-1887 antagonizes diabetic cachexia and decreases mortality. NPJ Aging Mech. Dis. 2017, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc. Diabetol. 2017, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Tanaka, Y.; Koiwai, K.; Inoue, K.; Hach, T.; Salsali, A.; Lund, S.S.; Broedl, U.C. Effect of empagliflozin monotherapy on postprandial glucose and 24-h glucose variability in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc. Diabetol. 2015, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Llopis, S.; Bañuls, C.; de Marañon, A.M.; Diaz-Morales, N.; Jover, A.; Garzon, S.; Rocha, M.; Victor, V.M.; Hernandez-Mijares, A. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med. 2017, 108, 155–162. [Google Scholar] [CrossRef]
- Van Exel, E.; Gussekloo, J.; de Craen, A.J.; Frölich, M.; Bootsma-Van Der Wiel, A.; Westendorp, R.G. Leiden 85 Plus Study. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: The Leiden 85-Plus Study. Diabetes 2002, 51, 1088–1092. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Baldus, S.; Heeschen, C.; Meinertz, T.; Zeiher, A.M.; Eiserich, J.P.; Münzel, T.; Simoons, M.L.; Hamm, C.W.; CAPTURE Investigators. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 2003, 108, 1440–1445. [Google Scholar] [CrossRef]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Martinez de Marañon, A.; Rios-Navarro, C.; Alvarez, A.; Gomez, M.; Rocha, M.; Hernández-Mijares, A. Insulin resistance in PCOS patients enhances oxidative stress and leukocyte adhesion: Role of myeloperoxidase. PLoS ONE 2016, 11, e0151960. [Google Scholar] [CrossRef] [PubMed]
- Rovira-Llopis, S.; Rocha, M.; Falcon, R.; de Pablo, C.; Alvarez, A.; Jover, A.; Hernandez-Mijares, A.; Victor, V.M. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxid Redox Signal. 2013, 19, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, M.; Pulakazhi Venu, V.K.; Saifeddine, M.; Mihara, K.; Kang, S.; Fedak, P.W.M.; Alston, L.A.; Hirota, S.A.; Ding, H.; Triggle, C.R.; et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul. Pharmacol. 2018, 109, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef]
- Hattori, S. Anti-inflammatory effects of empagliflozin in patients with type 2 diabetes and insulin resistance. Diabetol. Metab. Syndr. 2018, 10, 93. [Google Scholar] [CrossRef]
- Hadjadj, S.; Rosenstock, J.; Meinicke, T.; Woerle, H.J.; Broedl, U.C. Initial Combination of Empagliflozin and Metformin in Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 1718–1728. [Google Scholar] [CrossRef]



| Baseline | 12 Weeks Empagliflozin | 24 Weeks Empagliflozin | p-Value | |
|---|---|---|---|---|
| N (males) | 15 (11) | 15 (11) | 15 (11) | |
| Age (years) | 60.8 ± 10.2 | - | - | |
| Weight (kg) | 88.1 ± 19.8 | 85.1 ± 20.2 * | 83.7 ± 20.4 *# | <0.001 |
| Waist circumference (cm) | 104.4 ± 12.4 | 100.9 ± 15.6 | 98.22 ± 13.0 ** | <0.05 |
| SBP (mmHg) | 139.3 ± 25.9 | 137.3 ± 20.9 | 139.3 ± 21.2 | ns |
| DBP (mmHg) | 77.2 ± 11.6 | 79.1 ± 13.3 | 77.0 ± 12.5 | ns |
| Total cholesterol (mg/dL) | 141 ± 25 | 155 ± 28 * | 150 ± 27 | <0.05 |
| LDL-c (mg/dL) | 79.5 ± 18.6 | 89.2 ± 17.0 | 86.7 ± 20.4 | ns |
| HDL-c (mg/dL) | 44.6 ± 7.8 | 42.8 ± 6.9 | 47.0 ± 3.9 | ns |
| Triglycerides (mg/dL) | 94 (86−137) | 119 (101−171) * | 108 (85−130) | <0.05 |
| Insulin (μUI/mL) | 9.6 ± 5.7 | 9.3 ± 5.8 | 9.2 ± 6.1 | ns |
| HOMA-IR | 3.28 ± 2.23 | 3.00 ± 1.63 | 2.83 ± 1.96 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannantuoni, F.; M. de Marañon, A.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. https://doi.org/10.3390/jcm8111814
Iannantuoni F, M. de Marañon A, Diaz-Morales N, Falcon R, Bañuls C, Abad-Jimenez Z, Victor VM, Hernandez-Mijares A, Rovira-Llopis S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. Journal of Clinical Medicine. 2019; 8(11):1814. https://doi.org/10.3390/jcm8111814
Chicago/Turabian StyleIannantuoni, Francesca, Aranzazu M. de Marañon, Noelia Diaz-Morales, Rosa Falcon, Celia Bañuls, Zaida Abad-Jimenez, Victor M. Victor, Antonio Hernandez-Mijares, and Susana Rovira-Llopis. 2019. "The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes" Journal of Clinical Medicine 8, no. 11: 1814. https://doi.org/10.3390/jcm8111814
APA StyleIannantuoni, F., M. de Marañon, A., Diaz-Morales, N., Falcon, R., Bañuls, C., Abad-Jimenez, Z., Victor, V. M., Hernandez-Mijares, A., & Rovira-Llopis, S. (2019). The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. Journal of Clinical Medicine, 8(11), 1814. https://doi.org/10.3390/jcm8111814