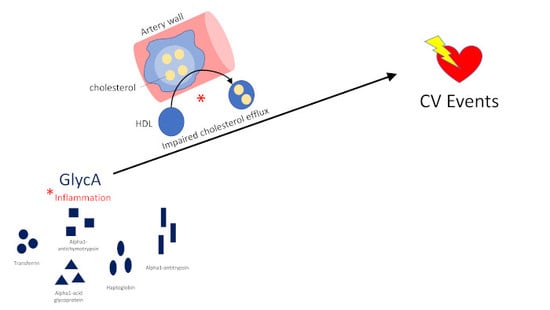
Impaired HDL Metabolism Links GlycA, A Novel Inflammatory Marker, with Incident Cardiovascular Events
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Assessment of HDL Parameters and GlycA
2.3. Clinical End Points
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Univariate Correlations with GlycA
3.3. Understanding Variation in GlycA
3.4. GlycA, HDL Parameters, and Incident Cardiovascular Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tehrani, D.M.; Zhao, Y.; Blaha, M.J.; Mora, S.; Mackey, R.H.; Michos, E.D.; Wong, N.D. Discordance of Low-Density Lipoprotein and High-Density Lipoprotein Cholesterol Particle Versus Cholesterol Concentration for the Prediction of Cardiovascular Disease in Patients with Metabolic Syndrome and Diabetes Mellitus (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2016, 117, 1921–1927. [Google Scholar] [CrossRef] [Green Version]
- Mora, S.; Glynn, R.J.; Ridker, P.M. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation 2013, 128, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Mackey, R.H.; Greenland, P.; Goff, D.C., Jr.; Lloyd-Jones, D.; Sibley, C.T.; Mora, S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J. Am. Coll. Cardiol. 2012, 60, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Neeland, I.J.; Das, S.R.; Khera, A.; Turer, A.T.; Ayers, C.R.; Rohatgi, A. Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas Heart Study). Am. J. Cardiol. 2015, 115, 890–894. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Shaul, P.W. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Vaisar, T.; Tang, C.; Babenko, I.; Hutchins, P.; Wimberger, J.; Suffredini, A.F.; Heinecke, J.W. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J. Lipid Res. 2015, 56, 1519–1530. [Google Scholar] [CrossRef] [Green Version]
- McGillicuddy, F.C.; de la Llera Moya, M.; Hinkle, C.C.; Joshi, M.R.; Chiquoine, E.H.; Billheimer, J.T.; Reilly, M.P. Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009, 119, 1135–1145. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Qin, S.; Dang, L.; Song, G.; Yao, S.; Yang, N.; Li, Y. Psoriasis decreases the anti-oxidation and anti-inflammation properties of high-density lipoprotein. Biochim. Biophys. Acta 2014, 1841, 1709–1715. [Google Scholar] [CrossRef]
- De la Llera Moya, M.; McGillicuddy, F.C.; Hinkle, C.C.; Byrne, M.; Joshi, M.R.; Nguyen, V.; Mehta, N.N. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis 2012, 222, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Akinkuolie, A.O.; Buring, J.E.; Ridker, P.M.; Mora, S. A novel protein glycan biomarker and future cardiovascular disease events. J. Am. Heart Assoc. 2014, 3, e001221. [Google Scholar] [CrossRef] [Green Version]
- Akinkuolie, A.O.; Glynn, R.J.; Padmanabhan, L.; Ridker, P.M.; Mora, S. Circulating N-Linked Glycoprotein Side-Chain Biomarker, Rosuvastatin Therapy, and Incident Cardiovascular Disease: An Analysis from the JUPITER Trial. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruppen, E.G.; Riphagen, I.J.; Connelly, M.A.; Otvos, J.D.; Bakker, S.J.; Dullaart, R.P. GlycA, a Pro-Inflammatory Glycoprotein Biomarker, and Incident Cardiovascular Disease: Relationship with C-Reactive Protein and Renal Function. PLoS ONE 2015, 10, e0139057. [Google Scholar] [CrossRef] [PubMed]
- Duprez, D.A.; Otvos, J.; Sanchez, O.A.; Mackey, R.H.; Tracy, R.; Jacobs, D.R., Jr. Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clin. Chem. 2016, 62, 1020–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarrah, R.W.; Kelly, J.P.; Craig, D.M.; Haynes, C.; Jessee, R.C.; Huffman, K.M.; Shah, S.H. A Novel Protein Glycan-Derived Inflammation Biomarker Independently Predicts Cardiovascular Disease and Modifies the Association of HDL Subclasses with Mortality. Clin. Chem. 2017, 63, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Guyton, J.R.; Connelly, M.A.; Akapame, S.; Bittner, V.; Kopecky, S.L.; Boden, W.E. Relations of GlycA and lipoprotein particle subspecies with cardiovascular events and mortality: A post hoc analysis of the AIM-HIGH trial. J. Clin. Lipidol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Gruppen, E.G.; Kunutsor, S.K.; Kieneker, L.M.; van der Vegt, B.; Connelly, M.A.; de Bock, G.H.; Dullaart, R.P. GlycA, a novel pro-inflammatory glycoprotein biomarker is associated with mortality: Results from the PREVEND study and meta-analysis. J. Intern. Med. 2019, 286, 596–609. [Google Scholar] [CrossRef]
- Otvos, J.D.; Shalaurova, I.; Wolak-Dinsmore, J.; Connelly, M.A.; Mackey, R.H.; Stein, J.H.; Tracy, R.P. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin. Chem. 2015, 61, 714–723. [Google Scholar] [CrossRef] [Green Version]
- The Davidson/Shah Lab. HDL Proteome Watch. Available online: http://homepages.uc.edu/~davidswm/HDL proteome.html (accessed on 19 November 2019).
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; Marsche, G. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.M.; Deng, J.; Tomann, A.B.; Shah, A.S.; Lu, L.J.; Davidson, W.S. Multi-dimensional co-separation analysis reveals protein-protein interactions defining plasma lipoprotein subspecies. Mol. Cell Proteom. 2013, 12, 3123–3134. [Google Scholar] [CrossRef] [Green Version]
- Gornik, O.; Lauc, G. Glycosylation of serum proteins in inflammatory diseases. Dis. Markers 2008, 25, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Ceciliani, F.; Pocacqua, V. The acute phase protein alpha1-acid glycoprotein: A model for altered glycosylation during diseases. Curr. Protein Pept. Sci. 2007, 8, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Pennathur, S.; Green, P.S.; Gharib, S.A.; Hoofnagle, A.N.; Cheung, M.C.; Chea, H. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Investig. 2007, 117, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; Dikkers, A.; De Boer, J.F.; Van Greevenbroek, M.M.; Van Der Kallen, C.J.; Schalkwijk, C.G.; Tietge, U.J. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: The CODAM study. Sci. Rep. 2016, 6, 27367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victor, R.G.; Haley, R.W.; Willett, D.L.; Peshock, R.M.; Vaeth, P.C.; Leonard, D.; Staab, J.M. The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am. J. Cardiol. 2004, 93, 1473–1480. [Google Scholar] [CrossRef]
- Maroules, C.D.; Rosero, E.; Ayers, C.; Peshock, R.M.; Khera, A. Abdominal aortic atherosclerosis at MR imaging is associated with cardiovascular events: The Dallas heart study. Radiology 2013, 269, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Rosenson, R.S.; Brewer, H.B.; Chapman, M.J.; Fazio, S.; Hussain, M.M.; Kontush, A.; Schaefer, E.J. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011, 57, 392–410. [Google Scholar] [CrossRef] [Green Version]


| Category | Mean +/− Standard Deviation |
|---|---|
| Overall (n = 2643) | 332 +/− 62 |
| Men (n = 1152) | 318 +/− 60 |
| Women (n = 1491) | 344 +/− 60 |
| White (n = 1305) | 337 +/− 64 |
| Black (n = 865) | 327 +/− 60 |
| Hispanic (n = 417) | 332 +/− 59 |
| Q1 170–291 μmol/L n = 600 Mean (SD) | Q2 292–327 μmol/L n = 603 Mean (SD) | Q3 328–369 μmol/L n = 597 Mean (SD) | Q4 370–660 μmol/L n = 592 Mean (SD) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 42 (9.4) | 44 (9.7) | 44 (9.6) | 45 (9.8) | <0.0001 |
| Systolic Blood Pressure (mmHg) | 120 (16.2) | 122 (17.4) | 125 (18.2) | 128 (19.7) | <0.0001 |
| BMI (kg/m2) | 27 (5.5) | 29 (5.7) | 30 (7.1) | 32 (8.0) | <0.0001 |
| Waist Circumference (cm) | 93 (15.0) | 98 (14.8) | 100 (17.0) | 104 (16.6) | <0.0001 |
| HOMA-IR | 3.2 (4.1) | 3.6 (3.1) | 4.6 (4.8) | 5.1 (4.5) | <0.0001 |
| Non-HDL (mg/dL) | 127 (39.1) | 130 (39.0) | 132 (39.9) | 136 (41.8) | 0.001 |
| Triglycerides (mg/dL) | 84 (59, 123) | 91 (69, 136) | 104 (72, 153) | 111 (78, 162) | <0.0001 |
| hs-CRP (mg/L) | 1.9 (2.0) | 3.4 (3.8) | 5.2 (4.8) | 9.5 (6.7) | <0.0001 |
| HDL-C (mg/dL) | 49.9 (14.6) | 49.6 (14.3) | 50.3 (13.9) | 50.2 (15.9) | 0.7600 |
| HDL-P (μmol/L) | 32.5 (5.5) | 33.0 (6.1) | 33.4 (5.8) | 34.3 (7.5) | <0.0001 |
| Cholesterol Efflux (normalized to pool) | 1.06 (0.33) | 1.01 (0.30) | 1.03 (0.33) | 1.02 (0.30) | 0.06 |
| Apo A1 (μmol/L) | 122.1 (27.0) | 125.4 (28.2) | 128.2 (28.0) | 132.3 (32.4) | <0.0001 |
| Variable | Standardized Estimate | p-Value |
|---|---|---|
| HDL-P | 0.08 | <0.0001 |
| Apo A1 | 0.29 | <0.0001 |
| Cholesterol Efflux | −0.06 | 0.0004 |
| HDL Medium | 0.10 | <0.0001 |
| HDL Large | −0.07 | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riggs, K.A.; Joshi, P.H.; Khera, A.; Singh, K.; Akinmolayemi, O.; Ayers, C.R.; Rohatgi, A. Impaired HDL Metabolism Links GlycA, A Novel Inflammatory Marker, with Incident Cardiovascular Events. J. Clin. Med. 2019, 8, 2137. https://doi.org/10.3390/jcm8122137
Riggs KA, Joshi PH, Khera A, Singh K, Akinmolayemi O, Ayers CR, Rohatgi A. Impaired HDL Metabolism Links GlycA, A Novel Inflammatory Marker, with Incident Cardiovascular Events. Journal of Clinical Medicine. 2019; 8(12):2137. https://doi.org/10.3390/jcm8122137
Chicago/Turabian StyleRiggs, Kayla A., Parag H. Joshi, Amit Khera, Kavisha Singh, Oludamilola Akinmolayemi, Colby R. Ayers, and Anand Rohatgi. 2019. "Impaired HDL Metabolism Links GlycA, A Novel Inflammatory Marker, with Incident Cardiovascular Events" Journal of Clinical Medicine 8, no. 12: 2137. https://doi.org/10.3390/jcm8122137
APA StyleRiggs, K. A., Joshi, P. H., Khera, A., Singh, K., Akinmolayemi, O., Ayers, C. R., & Rohatgi, A. (2019). Impaired HDL Metabolism Links GlycA, A Novel Inflammatory Marker, with Incident Cardiovascular Events. Journal of Clinical Medicine, 8(12), 2137. https://doi.org/10.3390/jcm8122137