Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Concentrations in Patients with Obesity and the Risk of Obstructive Sleep Apnea (OSA)
Abstract
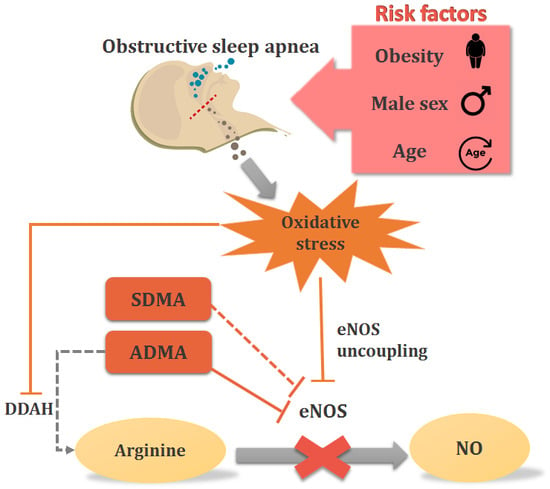
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Measurements of Biomarkers
2.3. Genotyping of the NOS3 4a/4b and NOS3 G894T Polymorphisms
2.4. ADMA, SDMA, and Arginine Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Shang, R.; Chen, Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide Biol. Chem. 2018, 78, 113–120. [Google Scholar] [CrossRef]
- Tsikas, D.; Boger, R.H.; Sandmann, J.; Bode-Boger, S.M.; Frolich, J.C. Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett. 2000, 478, 1–3. [Google Scholar] [CrossRef]
- Sasser, J.M.; Moningka, N.C.; Cunningham, M.W., Jr.; Croker, B.; Baylis, C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R740–R746. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.; Boger, R.; Luneburg, N. ADMA and the role of the genes: lessons from genetically modified animals and human gene polymorphisms. Pharmacol. Res. 2009, 60, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.; Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999, 43, 542–548. [Google Scholar] [CrossRef]
- Xuan, C.; Liu, Z.F.; Wang, Q.; Guo, F.F.; Zhang, X.; He, G.W.; Lun, L.M. Increased serum concentrations of asymmetric dimethylarginine (ADMA) in patients with early-onset coronary artery disease. Clin. Chim. Acta 2017, 464, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Sokal, M.; Sawicka, A.; Wlodarczyk, M.; Glowala, M.; Wrzosek, M.; Kosior, M.; Talalaj, M.; Biecek, P.; Nowicka, G. Impact of obesity and nitric oxide synthase gene G894T polymorphism on essential hypertension. J. Physiol. Pharmacol. 2015, 66, 681–689. [Google Scholar]
- Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Mbata, G.; Chukwuka, J. Obstructive sleep apnea hypopnea syndrome. Ann. Med. Health Sci. Res. 2012, 2, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqbali, S.R.; Mula-Abed, W.A. Comparison between Three Different Equations for the Estimation of Glomerular Filtration Rate in Omani Patients with Type 2 Diabetes Mellitus. Sultan Qaboos Univ. Med J. 2014, 14, e197–e203. [Google Scholar] [PubMed]
- Desjardins, F.; Balligand, J.L. Nitric oxide-dependent endothelial function and cardiovascular disease. Acta Clin. Belg. 2006, 61, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Dobutovic, B.; Smiljanic, K.; Soskic, S.; Düngen, H.-D.; Isenovi, E.R. Nitric Oxide and Its Role in Cardiovascular Diseases. Open Nitric Oxide J. 2011, 3, 65–71. [Google Scholar] [CrossRef]
- Koren, D.; Chirinos, J.A.; Katz, L.E.; Mohler, E.R.; Gallagher, P.R.; Mitchell, G.F.; Marcus, C.L. Interrelationships between obesity, obstructive sleep apnea syndrome and cardiovascular risk in obese adolescents. Int. J. Obes. 2015, 39, 1086–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoto, B.; Parlongo, R.M.; Parlongo, G.; Sgro, E.; Zoccali, C. The enzymatic machinery for ADMA synthesis and degradation is fully expressed in human adipocytes. J. Nephrol. 2007, 20, 554–559. [Google Scholar]
- Sydow, K.; Fortmann, S.P.; Fair, J.M.; Varady, A.; Hlatky, M.A.; Go, A.S.; Iribarren, C.; Tsao, P.S. Distribution of asymmetric dimethylarginine among 980 healthy, older adults of different ethnicities. Clin. Chem. 2010, 56, 111–120. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Yan, X.; Yan, F.; Sun, Y.; Zeng, J.; Waigel, S.; Yin, Y.; Fraig, M.M.; Egilmez, N.K.; et al. Circulating Adipose Fatty Acid Binding Protein Is a New Link Underlying Obesity-Associated Breast/Mammary Tumor Development. Cell Metab. 2018, 28, 689–705. [Google Scholar] [CrossRef]
- Mangiacapra, F.; Conte, M.; Demartini, C.; Muller, O.; Delrue, L.; Dierickx, K.; Di Sciascio, G.; Trimarco, B.; De Bruyne, B.; Wijns, W.; et al. Relationship of asymmetric dimethylarginine (ADMA) with extent and functional severity of coronary atherosclerosis. Int. J. Cardiol. 2016, 220, 629–633. [Google Scholar] [CrossRef]
- Xuan, C.; Tian, Q.W.; Li, H.; Zhang, B.B.; He, G.W.; Lun, L.M. Levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, and risk of coronary artery disease: A meta-analysis based on 4713 participants. Eur. J. Prev. Cardiol. 2016, 23, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Triches, C.B.; Mayer, S.; Quinto, B.M.R.; Batista, M.C.; Zanella, M.T. Association of endothelial dysfunction with cardiovascular risk factors and new-onset diabetes mellitus in patients with hypertension. J. Clin. Hypertens. 2018, 20, 935–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciacqua, A.; Grillo, N.; Quero, M.; Sesti, G.; Perticone, F. Asymmetric dimethylarginine plasma levels and endothelial function in newly diagnosed type 2 diabetic patients. Int. J. Mol. Sci. 2012, 13, 13804–13815. [Google Scholar] [CrossRef] [PubMed]
- Hammes, M.S.; Watson, S.; Coe, F.L.; Ahmed, F.; Beltran, E.; Dhar, P. Asymmetric dimethylarginine and whole blood viscosity in renal failure. Clin. Hemorheol. Microcirc. 2015, 59, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelaert, J.; Schepers, E.; Glorieux, G.; Eloot, S.; Vanholder, R.; Lynen, F. Determination of Asymmetric and Symmetric Dimethylarginine in Serum from Patients with Chronic Kidney Disease: UPLC-MS/MS versus ELISA. Toxins 2016, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Glantz, H.; Thunstrom, E.; Herlitz, J.; Cederin, B.; Nasic, S.; Ejdeback, J.; Peker, Y. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann. Am. Thorac. Soc. 2013, 10, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Genta, P.R.; Pedrosa, R.P.; Nerbass, F.B.; Gonzaga, C.C.; Krieger, E.M.; Lorenzi-Filho, G. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am. J. Cardiol. 2010, 105, 1135–1139. [Google Scholar] [CrossRef]
- Badran, M.; Abuyassin, B.; Golbidi, S.; Ayas, N.; Laher, I. Uncoupling of Vascular Nitric Oxide Synthase Caused by Intermittent Hypoxia. Oxidative Med. Cell. Longev. 2016, 2016, 2354870. [Google Scholar] [CrossRef]
- Tsioufis, C.; Dimitriadis, K.; Andrikou, E.; Thomopoulos, C.; Tsiachris, D.; Stefanadi, E.; Mihas, C.; Miliou, A.; Papademetriou, V.; Stefanadis, C. ADMA, C-reactive protein, and albuminuria in untreated essential hypertension: A cross-sectional study. Am. J. Kidney Dis. 2010, 55, 1050–1059. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Hill, M.A.; Wu, J. Does C-reactive protein contribute to atherothrombosis via oxidant-mediated release of pro-thrombotic factors and activation of platelets? Front. Physiol. 2012, 3, 433. [Google Scholar] [CrossRef]
- Thomopoulos, C.; Tsioufis, C.; Dimitriadis, K.; Tsiachris, D.; Tousoulis, D.; Manolis, A.; Alchanatis, M.; Kallikazaros, I.; Stefanadis, C. Obstructive sleep apnoea syndrome is associated with enhanced sub-clinical inflammation and asymmetric dimethyl-arginine levels in hypertensives. J. Hum. Hypertens. 2008, 23, 65–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korandji, C.; Zeller, M.; Guilland, J.C.; Vergely, C.; Sicard, P.; Duvillard, L.; Gambert, P.; Moreau, D.; Cottin, Y.; Rochette, L. Asymmetric dimethylarginine (ADMA) and hyperhomocysteinemia in patients with acute myocardial infarction. Clin. Biochem. 2007, 40, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Schwedhelm, E.; Arakawa, N.; Bode-Böger, S.M.; Tsikas, D.; Hornig, B.; Frölich, J.; Böger, R.H. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc. Res. 2003, 57, 244–252. [Google Scholar] [CrossRef]
- Yoo, J.H.; Lee, S.C. Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke. Atherosclerosis 2001, 158, 425–430. [Google Scholar] [CrossRef]
- Doshi, S.; McDowell, I.; Goodfellow, J.; Stabler, S.; Boger, R.; Allen, R.; Newcombe, R.; Lewis, M.; Moat, S. Relationship between S-adenosylmethionine, S-adenosylhomocysteine, asymmetric dimethylarginine, and endothelial function in healthy human subjects during experimental hyper- and hypohomocysteinemia. Metabolism 2005, 54, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Boger, R.H.; Lentz, S.R.; Bode-Boger, S.M.; Knapp, H.R.; Haynes, W.G. Elevation of asymmetrical dimethylarginine may mediate endothelial dysfunction during experimental hyperhomocyst(e)inaemia in humans. Clin. Sci. 2001, 100, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Perticone, F.; Sciacqua, A.; Maio, R.; Perticone, M.; Maas, R.; Boger, R.H.; Tripepi, G.; Sesti, G.; Zoccali, C. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J. Am. Coll. Cardiol. 2005, 46, 518–523. [Google Scholar] [CrossRef]
- Sonmez, A.; Celebi, G.; Erdem, G.; Tapan, S.; Genc, H.; Tasci, I.; Ercin, C.N.; Dogru, T.; Kilic, S.; Uckaya, G.; et al. Plasma apelin and ADMA Levels in patients with essential hypertension. Clin. Exp. Hypertens. 2010, 32, 179–183. [Google Scholar] [CrossRef]


| Variables | Mean ± SD | Median (IQR) | * Rho/p-Value | |||
|---|---|---|---|---|---|---|
| ADMA | SDMA | Arginine | Ratio Arginine/ADMA | |||
| ADMA (µmol/L) | 0.5 ± 0.1 | 0.5 (0.5–0.6) | - | 0.48/<0.0001 | 0.21/<0.0001 | −0.45/<0.0001 |
| SDMA (µmol/L) | 1.3 ± 0.1 | 1.3 (1.1–1.5) | 0.48/<0.0001 | - | 0.01/0.7894 | −0.31/<0.0001 |
| Arginine (µmol/L) | 105.87 ± 38.9 | 99.31 (86.11–115.67) | 0.21/<0.0001 | 0.01/0.7894 | - | 0.73/<0.0001 |
| Ratio Arg/ADMA | 177.3 ± 66.3 | 165.2 (139.1–197.3) | −0.45/0.0001 | −0.31/<0.0001 | 0.73/<0.0001 | - |
| Age in years | 43.8 ± 11.3 | 43.0 (35.0–53.0) | −0.01/0.8345 | 0.19/<0.0001 | −0.14/0.0016 | −0.12/0.0076 |
| Weight (kg) | 121.4 ± 23.6 | 119.0 (103.5–134.0) | 0.17/0.0001 | 0.15/0.0005 | 0.04/0.3165 | −0.07/0.0934 |
| BMI | 42.6 ± 6.7 | 41.6 (37.7–46.4) | 0.23/0.0001 | 0.15/0.0008 | 0.04/0.3352 | −0.11/0.1070 |
| Fat % | 42.8 ± 6.8 | 44.2 (39.1–47.4) | 0.12/0.0161 | 0.07/0.1335 | 0.03/0.5140 | −0.04/0.3201 |
| Fat mass (kg) | 50.7 ± 7.9 | 48.12 (40.68–5.49) | 0.09/0.0799 | 0.08/0.1153 | 0.09/0.0529 | 0.01/0.8573 |
| Systolic blood pressure (mmHg) | 133.9 ± 17.8 | 132.0 (120.0–143.0) | 0.18/<0.0001 | 0.06/0.1944 | −0.12/0.0081 | −0.22/<0.0001 |
| Diastolic blood pressure (mmHg) | 76.6 ± 10.6 | 76.0 (70.0–81.0) | 0.05/0.2091 | −0.02/0.6255 | −0.08/0.0692 | −0.10/0.0238 |
| Fasting glucose (mg/dL) | 108.1 ± 36.3 | 98.0 (88.0–114.0) | −0.08/0.0796 | 0.09/0.0388 | −0.07/0.0901 | −0.01/0.9032 |
| HbA1c (%) | 6.2 ± 1.3 | 5.9 (5.4–6.5) | 0.17/<0.0001 | 0.16/0.0002 | −0.08/0.0716 | 0.05/0.2368 |
| Total cholesterol (mg/dL) | 184.6 ± 45.1 | 183.0 (155.0–210.0) | −0.06/0.1421 | 0.10/0.0213 | −0.11/0.0164 | −0.15/0.0006 |
| LDL-cholesterol (mg/dL) | 110.2 ± 35.7 | 110.0 (84.0–133.0) | −0.06/0.1533 | 0.10/0.0241 | −0.11/0.0157 | −0.15/0.0009 |
| HDL-cholesterol (mg/dL) | 42.8 ± 11.6 | 41.9 (35.0–49.0) | 0.03/0.4388 | −0.14/0.0018 | −0.02/0.6179 | −0.03/0.4563 |
| Triglycerides (mg/dL) | 160.2 ± 144.9 | 138.0 (100.0–188.0) | −0.02/0.5974 | 0.13/0.0033 | 0.03/0.4505 | 0.05/0.2355 |
| Alanine aminotransferase (U/L) | 51.3 ± 46.5 | 43.0 (33.0–58.0) | −0.04/0.3025 | −0.05/0.2933 | 0.01/0.8602 | 0.04/0.3402 |
| Aspartate aminotransferase (U/L) | 30.1 ± 29.3 | 24.0 (19.0–32.0) | 0.02/0.5842 | 0.01/0.7619 | −0.03/0.4632 | −0.04/0.3861 |
| CRE (mg/dL) | 0.86 ±0.2 | 0.83 (0.73–0.95) | 0.02/0.7000 | 0.37/<0.0001 | −0.02/0.6217 | −0.02/0.6742 |
| B12 (pg/mL) | 334.5 ± 161.4 | 302.0 (245.0–380.0) | −0.08/0.0669 | −0.10/0.0177 | −0.03/0.4883 | 0.02/0.7315 |
| Folic acid (ng/mL) | 8.2 ± 3.6 | 7.5 (5.6–10.2) | −0.20/<0.0001 | −0.16/0.0002 | −0.04/0.2902 | 0.10/0.0241 |
| Apnea Hypopnea Index | 14.2 ± 21.2 | 5.5 (1.5–16.1) | 0.14/0.0024 | 0.16/0.0005 | −0.02/0.6504 | −0.09/0.0481 |
| IL-6 (pg/mL) | 3.5 ± 4.7 | 2.5 (1.4–4.3) | 0.15/0.0016 | 0.14/0.0032 | −0.01/0.8205 | −0.11/0.0166 |
| ESR | 17.7 ± 13.6 | 15.0 (8.0–24.0) | −0.02/0.6915 | −0.07/0.1147 | 0.17/<0.0001 | 0.16/0.0003 |
| CRP (g/L) | 9.2 ± 12.5 | 6.4 (3.0–12.2) | 0.17/0.0001 | 0.06/0.1488 | 0.02/0.6671 | −0.10/0.0247 |
| eGFR (CKD-EPI) mL/min/1.73 m2 | 92.0 ± 17.4 | 94.1 (80.4–104.6) | 0.02/0.6020 | −0.33/<0.0001 | 0.07/0.1049 | 0.04/0.3704 |
| eGFR (MDRD) mL/min/1.73 m2 | 88.1 ± 18.9 | 87.6 (76.0–98.7) | 0.02/0.6875 | −0.31/<0.0001 | 0.04/0.3427 | 0.02/0.7079 |
| N | ADMA, µmol/L | SDMA, µmol/L | Arginine, µmol/L | Ratio Arginine/ADMA, µmol/L | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| BMI Level | |||||||||
| I BMI 30.0–34.9 | 47 | 0.49 ± 0.09 | 0.49 (0.42–0.53) | 1.24 ± 0.29 | 1.22 (1.06–1.38) | 97.58 ± 21.54 | 91.27 (82.66–108.78) | 176.75± 45.44 | 171.43 (141.44–200.94) |
| II BMI 35–39.9 | 148 | 0.51 ± 0.09 | 0.50 (0.45–0.56) | 1.31 ± 0.37 | 1.27 (1.11–1.44) | 103.68 ± 36.76 | 97.30 (86.68–112.80) | 179.20± 65.85 | 168.34 (147.66–197.21) |
| III BMI ≥ 40 | 323 | 0.54 ± 0.09 | 0.53 (0.48-0.93) | 1.35 ± 0.30 | 1.30 (1.15–1.51) | 108.02 ± 41.60 | 102.18 (86.68–118.26) | 176.40± 69.12 | 163.86 (138.02–197.16) |
| Statistics (Anova/Kruskal-Wallis tests) | F = 9.89; df = 2; p < 0.0001 | p < 0.0001 | F = 3.13; df = 2; p = 0.0443 | p = 0.0113 | F = 1.80; df = 2; p = 0.1664 | p = 0.0716 | F = 0.09; df = 2; p = 0.9126 | p = 0.489 | |
| Hypertension | |||||||||
| No | 169 | 0.52 ± 0.08 | 0.51 (0.47–0.56) | 1.24 ± 0.29 | 1.27 (1.11–1.40) | 110.20 ± 45.15 | 102.18 (90.70–117.68) | 184.92± 75.84 | 171.30 (147.57–203.84) |
| Yes | 349 | 0.53 ± 0.09 | 0.52 (0.46–0.59) | 1.31 ± 0.37 | 1.34 (1.17–1.57) | 103.78 ± 35.33 | 98.16 (83.81–114.24) | 173.66 ± 60.86 | 164.23 (137.62–195.19) |
| Statistics (t-test/Mann-Whitney test) | p = 0.5904; t = 0.54 | p = 0.6074 | p = 0.0176; t = 2.40 | p = 0.0500 | p = 0.0781; t =−1.76 | p = 0.0152 | p = 0.0697; t =−1.82 | p = 0.0325 | |
| AHI Level | |||||||||
| AHI < 5 | 276 | 0.51 ± 0.09 | 0.50 (0.45–0.57) | 1.29 ± 0.33 | 1.27 (1.11–1.40) | 106.31 ± 37.22 | 99.31 (86.68–116.53) | 181.83 ± 62.73 | 173.85 (140.16–206.75) |
| AHI ≥ 5 | 242 | 0.54 ± 0.09 | 0.53 (0.48–0.59) | 1.37 ± 0.32 | 1.32 (1.17–1.57) | 103.53 ± 41.05 | 100.17 (86.68–113.09) | 173.74 ± 68.39 | 159.56 (138.71–191.45) |
| Statistics (t-test/Mann-Whitney test) | p = 0.0036; t = −2.93 | p = 0.00072 | p = 0.0041; t = −2.89 | p = 0.00117 | p = 0.9508; t = −0.06 | p = 0.6749 | p = 0.1878; t = 1.32 | p = 0.0168 | |
| N | ADMA, µmol/L | SDMA, µmol/L | Arginine, µmol/L | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Genotype NOS3 G894T | |||||||
| GT | 150 | 0.50 ± 0.09 | 0.49 (0.45-0.55) | 1.28 ± 0.27 | 1.24 (1.09–1.44) | 104.38 ± 30.25 | 102.18 (86.68–115.96) |
| TT | 83 | 0.53 ± 0.09 | 0.51 (0.46–0.59) | 1.40 ± 0.47 | 1.32 (1.13–1.53) | 106.13 ± 46.30 | 95.87 (83.24–114.24) |
| GG | 285 | 0.53± 0.09 | 0.53 (0.47–0.59) | 1.34 ± 0.29 | 1.29 (1.15–1.46) | 106.59 ± 40.70 | 99.60 (86.68–116.53) |
| Statistics (Anova/Kruskal-Wallis tests) | F = 6.13; df = 2; p = 0.0023 | p = 0.0019 | F = 3.77; df = 2; p = 0.0237 | p = 0.0630 | F = 0.16; df = 2; p = 0.8515 | p = 0.3947 | |
| Genotype NOS3 4a/4b | |||||||
| 4a/4a | 19 | 0.50 ± 0.08 | 0.51 (0.44–0.55) | 1.30 ± 0.28 | 1.33 (1.09–1.47) | 106.47 ± 25.97 | 99.31 (88.98–116.53) |
| 4a/4b | 158 | 0.54 ± 0.10 | 0.53 (0.47–0.59) | 1.35 ± 0.29 | 1.31 (1.16–1.49) | 109.66 ± 44.49 | 102.18 (86.11–117.11) |
| 4b/4b | 341 | 0.52 ± 0.08 | 0.51 (0.46–0.58) | 1.32 ± 0.33 | 1.28 (1.11–1.45) | 104.17 ± 36.61 | 98.74 (86.11–114.24) |
| Statistics (Anova/Kruskal-Wallis tests) | F = 2.29; df = 2; p = 0.1020 | p = 0.3073 | F = 0.41; df = 2; p = 0.6670 | p = 0.4418 | F = 1.08; df = 2; p = 0.3410 | p = 0.401 | |
| Variables | OSA | Model 1 a OR (95% CI) | pa | Model 2 b OR (95% CI) | pb | |
|---|---|---|---|---|---|---|
| AHI < 5 n = 276 | AHI ≥ 5 n = 242 | |||||
| ADMA above median (>0.514 µmol/L), n (%) Yes No | 117 (42.5) 159 (57.6) | 135 (55.8) 107 (44.2) | 1.71 (1.21–2.43) | 0.002 | 1.74 (1.12–2.71) | 0.0132 |
| SDMA above median (>1.286 µmol/L), n (%) Yes No | 119 (43.0) 157 (57.0) | 132 (54.5) 110 (45.5) | 1.58 (1.12–2.24) | 0.010 | 1.15 (0.73–1.81) | 0.537 |
| Age above median (>43 years), n (%) Yes No | 97 (35.2) 179 (64.8) | 145 (59.9) 97 (40.1) | 2.76 (1.93–3.94) | <0.001 | 2.25 (1.42–3.56) | <0.001 |
| Hypertension, n (%) Yes No | 147 (53.3) 129 (46.7) | 187 (77.3) 55 (22.7) | 2.98 (2.03–4.37) | <0.001 | 1.77 (1.11–2.80) | 0.015 |
| BMI, n (%) above 40 30–39.9 | 149 (54.0) 127 (46.0) | 172 (71.0) 70 (29.0) | 2.09 (1.45–3.02) | <0.001 | 2.26 (1.50–3.50) | <0.001 |
| Sex, n (%) Male Female | 36 (13.0) 240 (87.0) | 98 (40.5) 144 (59.5) | 4.54 (2.94–7.00) | <0.001 | 3.82 (2.30–6.34) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arlouskaya, Y.; Sawicka, A.; Głowala, M.; Giebułtowicz, J.; Korytowska, N.; Tałałaj, M.; Nowicka, G.; Wrzosek, M. Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Concentrations in Patients with Obesity and the Risk of Obstructive Sleep Apnea (OSA). J. Clin. Med. 2019, 8, 897. https://doi.org/10.3390/jcm8060897
Arlouskaya Y, Sawicka A, Głowala M, Giebułtowicz J, Korytowska N, Tałałaj M, Nowicka G, Wrzosek M. Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Concentrations in Patients with Obesity and the Risk of Obstructive Sleep Apnea (OSA). Journal of Clinical Medicine. 2019; 8(6):897. https://doi.org/10.3390/jcm8060897
Chicago/Turabian StyleArlouskaya, Yana, Ada Sawicka, Marek Głowala, Joanna Giebułtowicz, Natalia Korytowska, Marek Tałałaj, Grażyna Nowicka, and Małgorzata Wrzosek. 2019. "Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Concentrations in Patients with Obesity and the Risk of Obstructive Sleep Apnea (OSA)" Journal of Clinical Medicine 8, no. 6: 897. https://doi.org/10.3390/jcm8060897
APA StyleArlouskaya, Y., Sawicka, A., Głowala, M., Giebułtowicz, J., Korytowska, N., Tałałaj, M., Nowicka, G., & Wrzosek, M. (2019). Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Concentrations in Patients with Obesity and the Risk of Obstructive Sleep Apnea (OSA). Journal of Clinical Medicine, 8(6), 897. https://doi.org/10.3390/jcm8060897