High N-Terminal proB-Type Natriuretic Peptide Indicates Elevated Risk of Death after Percutaneous Coronary Intervention Compared to Coronary Artery Bypass Surgery in Patients with Left Ventricular Dysfunction
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
- -
- To answer if there is an association between type of revascularization procedure and death, death was adjusted by age, sex, rhythm, prior myocardial infarction, diabetes mellitus (DM), estimated glomerular filtration rate (Cockcroft-Gault formula; eGFR), New York Heart Association (NYHA) class, Canadian Cardiovascular Society (CCS) class, number of coronary vessels diseased and revascularization procedure in Model A.
- -
- To answer if death is associated by levels of NT-proBNP, death was adjusted by age, sex, rhythm, prior myocardial infarction, DM, eGFR, NYHA class, CCS class, number of coronary vessels diseased and NT-proBNP in Model B.
- -
- To answer if there is an interaction between revascularization procedure and NT-proBNP, death was adjusted by age, sex, rhythm, prior myocardial infarction, DM, eGFR, NYHA class, CCS class, number of coronary vessels diseased, NT-proBNP, revascularization procedure, and NT-proBNP*revascularization procedure in Model C.
- -
- To confirm an association between type of revascularization procedure and death in patients with above median NT-proBNP, death was adjusted by age, sex, rhythm, prior myocardial infarction, DM, eGFR, NYHA class, CCS class, number of coronary vessels diseased, and revascularization procedure in Model D.
- -
- To confirm no association between type of revascularization procedure and death in patients with below median NT-proBNP, death was adjusted by age, sex, rhythm, prior myocardial infarction, DM, eGFR, NYHA class, CCS class, number of coronary vessels diseased, and revascularization procedure in Model E.
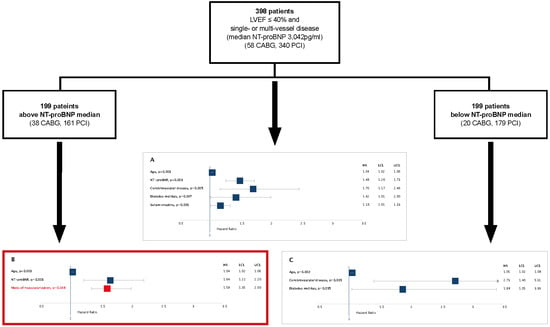
3. Results
3.1. Results of the Cox Proportional Hazards Regression Models
3.2. Relative Risk of Death of PCI Compared to CABG in Patients with Lower and Elevated NT-proBNP
4. Discussion
4.1. Comparison of Outcome after PCI and CABG
4.2. Mode of Death
4.3. Prothrombotic State and Inflammation
4.4. Limitations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alderman, E.L.; Fisher, L.D.; Litwin, P.; Kaiser, G.C.; Myers, W.O.; Maynard, C.; Levine, F.; Schloss, M. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation 1983, 68, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Chien, C.V.; Bidwell, J.T.; Gelow, J.M.; Denfeld, Q.E.; Masterson Creber, R.; Buck, H.G.; Mudd, J.O. Comorbidity profiles and inpatient outcomes during hospitalization for heart failure: An analysis of the U.S. Nationwide inpatient sample. BMC Cardiovasc. Disord. 2014, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Shahian, D.M.; O’Brien, S.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.L.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 1—Coronary artery bypass grafting surgery. Ann. Thorac. Surg. 2009, 88 (Suppl. 1), S2–S22. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Juni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [PubMed]
- Bangalore, S.; Guo, Y.; Samadashvili, Z.; Blecker, S.; Hannan, E.L. Revascularization in Patients with Multivessel Coronary Artery Disease and Severe Left Ventricular Systolic Dysfunction: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. Circulation 2016, 133, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Gioia, G.; Matthai, W.; Gillin, K.; Dralle, J.; Benassi, A.; Gioia, M.F.; White, J. Revascularization in severe left ventricular dysfunction: Outcome comparison of drug-eluting stent implantation versus coronary artery by-pass grafting. Catheter. Cardiovasc. Interv. 2007, 70, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Lee, C.W.; Baek, S.; Lee, P.H.; Ahn, J.M.; Park, D.W.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Park, S.W.; et al. Comparison of Outcomes of Coronary Artery Bypass Grafting Versus Drug-Eluting Stent Implantation in Patients with Severe Left Ventricular Dysfunction. Am. J. Cardiol. 2017, 120, 69–74. [Google Scholar] [CrossRef]
- Wolff, G.; Dimitroulis, D.; Andreotti, F.; Kolodziejczak, M.; Jung, C.; Scicchitano, P.; Devito, F.; Zito, A.; Occhipinti, M.; Castiglioni, B.; et al. Survival Benefits of Invasive Versus Conservative Strategies in Heart Failure in Patients with Reduced Ejection Fraction and Coronary Artery Disease: A Meta-Analysis. Circ. Heart Fail. 2017, 10, e003255. [Google Scholar] [CrossRef] [PubMed]
- Mongirdiene, A.; Kursvietiene, L.; Kasauskas, A. The coagulation system changes in patients with chronic heart failure. Medicina 2010, 46, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Clopton, P.; de Filippi, C.R.; Sanchez, O.A.; Bahrami, H.; Lima, J.A.; Tracy, R.P.; Siscovick, D.; Bertoni, A.G.; Greenland, P.; et al. Serial measurement of N-terminal pro-B-type natriuretic peptide and cardiac troponin T for cardiovascular disease risk assessment in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. Heart J. 2015, 170, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.; et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Moertl, D.; Peter, S.; Ahmadi, R.; Huelsmann, M.; Yamuti, S.; Wagner, B.; Pacher, R. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J. Am. Coll. Cardiol. 2010, 55, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Huelsman, M.; Strecker, K.; Bojic, A.; Moser, P.; Stanek, B.; Pacher, R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002, 105, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Pant, R.; Patel, M.; Garcia-Sayan, E.; Wassouf, M.; D’Silva, O.; Kehoe, R.F.; Doukky, R. Impact of B-type natriuretic peptide level on the risk of left atrial appendage thrombus in patients with nonvalvular atrial fibrillation: A prospective study. Cardiovasc. Ultrasound 2016, 14, 4. [Google Scholar] [CrossRef]
- Jose, J.; Sulimov, D.S.; El-Mawardy, M.; Sato, T.; Allali, A.; Holy, E.W.; Becker, B.; Landt, M.; Kebernik, J.; Schwarz, B.; et al. Clinical Bioprosthetic Heart Valve Thrombosis After Transcatheter Aortic Valve Replacement: Incidence, Characteristics, and Treatment Outcomes. JACC Cardiovasc. Interv. 2017, 10, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahim, A.H.; Perez, A.C.; Fulton, R.L.; Jhund, P.S.; Latini, R.; Tognoni, G.; Wikstrand, J.; Kjekshus, J.; Lip, G.Y.; Maggioni, A.P.; et al. Risk of Stroke in Chronic Heart Failure Patients Without Atrial Fibrillation: Analysis of the Controlled Rosuvastatin in Multinational Trial Heart Failure (CORONA) and the Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca-Heart Failure (GISSI-HF) Trials. Circulation 2015, 131, 1486–1494. [Google Scholar]
- Uretsky, B.F.; Thygesen, K.; Armstrong, P.W.; Cleland, J.G.; Horowitz, J.D.; Massie, B.M.; Packer, M.; Poole-Wilson, P.A.; Ryden, L. Acute coronary findings at autopsy in heart failure patients with sudden death: Results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation 2000, 102, 611–616. [Google Scholar] [CrossRef]
- Orn, S.; Cleland, J.G.; Romo, M.; Kjekshus, J.; Dickstein, K. Recurrent infarction causes the most deaths following myocardial infarction with left ventricular dysfunction. Am. J. Med. 2005, 118, 752–758. [Google Scholar] [CrossRef]
- van Werkum, J.W.; Heestermans, A.A.; Zomer, A.C.; Kelder, J.C.; Suttorp, M.J.; Rensing, B.J.; Koolen, J.J.; Brueren, B.R.; Dambrink, J.H.; Hautvast, R.W.; et al. Predictors of coronary stent thrombosis: The Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 2009, 53, 1399–1409. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Stough, W.G.; Regnault, V.; Gheorghiade, M.; Deliargyris, E.; Gibson, C.M.; Agewall, S.; Berkowitz, S.D.; Burton, P.; Calvo, G.; et al. Is thrombosis a contributor to heart failure pathophysiology? Possible mechanisms, therapeutic opportunities, and clinical investigation challenges. Int. J. Cardiol. 2013, 167, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Whincup, P.H.; Papacosta, O.; Lennon, L.; Lowe, G.D. Associations between blood coagulation markers, NT-proBNP and risk of incident heart failure in older men: The British Regional Heart Study. Int. J. Cardiol. 2017, 230, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Greenberg, B.; Cleland, J.G.; Gheorghiade, M.; van Veldhuisen, D.J.; Mehra, M.R.; Anker, S.D.; Byra, W.M.; Fu, M.; Mills, R.M. Rationale and design of a randomized, double-blind, event-driven, multicentre study comparing the efficacy and safety of oral rivaroxaban with placebo for reducing the risk of death, myocardial infarction or stroke in subjects with heart failure and significant coronary artery disease following an exacerbation of heart failure: The COMMANDER HF trial. Eur. J. Heart Fail. 2015, 17, 735–742. [Google Scholar] [PubMed]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed]


| All n = 398 | Above NT-pBNP Median (>3042 pg/mL) n = 199 | Below NT-pBNP Median (<3042 pg/mL) n = 199 | p-Value | Revascularization Procedure | p-Value | ||
|---|---|---|---|---|---|---|---|
| PCI n = 340 | CABG n = 58 | ||||||
| Age, years; median (IQR) | 67 (59/75) * | 70 (64/78) * | 64 (55/71) * | <0.001 | 66 (58/76) | 69 (61/73) | 0.404 |
| Gender | 0.526 | 0.661 | |||||
| Female; n (%) | 77 (19) | 41 (21) | 36 (18) | - | 67 (20) | 10 (17) | - |
| Male; n (%) | 321 (81) | 158 (7 9) | 163 (82) | - | 273 (80) | 48 (83) | - |
| Body-Mass-Index, kg/m2; median (IQR) | 27 (25/30) | 26 (24/29) | 28 (25/30) | 0.017 | 27 (25/30) | 27 (25/31) | 0.708 |
| Hypertension; n (%) | 306 (77) | 156 (78) | 150 (75) | 0.476 | 261 (77) | 45 (78) | 0.819 |
| Hyperlipidemia; n (%) | 295 (74) | 143 (72) | 152 (76) | 0.303 | 254 (75) | 41 (71) | 0.519 |
| Diabetes mellitus; n (%) | 168 (42) * | 95 (48) | 73 (37) † | 0.026 | 133 (39) | 35 (60) | 0.002 |
| Smoker | 0.021 | 0.850 | |||||
| Current; n (%) | 118 (30) | 53 (27) | 65 (33) | - | 102 (30) | 16 (28) | - |
| Previous; n (%) | 79 (20) | 32 (16) | 47 (24) | - | 66 (19) | 13 (22) | - |
| Family history; n (%) | 76 (19) | 31 (16) | 45 (23) | 0.074 | 67 (20) | 9 (16) | 0.453 |
| Cerebrovascular and/or peripheral vascular disease; n (%) | 124 (31) * | 86 (43) | 38 (19) † | <0.001 | 97 (29) | 27 (47) | 0.006 |
| Prior myocardial infarction; n (%) | 155 (39) ‡ | 76 (38) ‡ | 79 (40) | 0.758 | 135 (45) | 20 (35) | 0.451 |
| Prior PCI; n (%) | 94 (24) | 38 (19) | 56 (28) | 0.034 | 80 (24) | 14 (24) | 0.920 |
| Prior CABG; n (%) | 54 (14) | 21 (11) ‡ | 31 (16) | 0.242 | 53 (16) | 1 (2) | 0.004 |
| NYHA class | † | 0.076 | 0.800 | ||||
| Class I; n (%) | 81 (23) | 40 (22) | 41 (24) | - | 69 (22) | 12 (23) | - |
| Class II; n (%) | 55 (15) | 21 (11) | 34 (20) | - | 48 (16) | 7 (14) | - |
| Class III; n (%) | 168 (47) | 91 (49) | 77 (44) | - | 146 (47) | 22 (43) | - |
| Class IV; n (%) | 55 (15) | 34 (18) | 21 (12) | - | 45 (15) | 10 (20) | - |
| CCS class | ‡ | 0.252 | 0.303 | ||||
| Class I; n (%) | 61 (17) | 36 (19) | 25 (15) | - | 51 (17) | 10 (20) | - |
| Class II; n (%) | 77 (21) | 33 (18) | 44 (25) | - | 69 (22) | 8 (16) | - |
| Class III; n (%) | 127 (36) | 69 (37) | 58 (34) | - | 112 (36) | 15 (29) | - |
| Class IV; n (%) | 94 (26) | 48 (26) | 46 (27) | - | 76 (25) | 18 (35) | - |
| Elective/NSTEMI | 0.128 | 0.915 | |||||
| Elective; n (%) | 169 (43) | 77 (39) | 92 (46) | - | 144 (42) | 25 (43) | - |
| NSTEMI; n (%) | 229 (57) | 122 (61) | 107 (54) | - | 196 (58) | 33 (57) | - |
| All n = 398 | Above NT-pBNP Median (>3042 pg/mL) n = 199 | Below NT-pBNP Median (<3042 pg/mL) n = 199 | p-Value | Revascularization Procedure | p-Value | ||
|---|---|---|---|---|---|---|---|
| PCI n = 340 | CABG n = 58 | ||||||
| ACE-inhibitor/ARB; n (%) | 398 (100) | 199 (100) | 199 (100) | - | 340 (100) | 58 (100) | - |
| Beta-Bocker; n (%) | 316 (79) | 156 (78) | 160 (80) | 0.620 | 271 (80) | 45 (78) | 0.712 |
| Aldosteron; n (%) | 198 (50) | 106 (53) | 92 (46) | 0.160 | 168 (49) | 26 (45) | 0.417 |
| Aspirin; n (%) | 398 (100) | 199 (100) | 199 (100) | - | 340 (100) | 58 (100) | - |
| Clopidogrel; n (%) | 315 (79) | 152 (76) | 163 (82) | 0.175 | 315 (93) | - | - |
| Ticagrelor; n (%) | 25 (6) | 9 (5) | 16 (8) | 0.148 | 25 (7) | - | - |
| Pacemaker, n (%) | 29 (7) | 18 (9) | 11 (6) | 0.177 | 28 (8) | 1 (2) | 0.078 |
| ICD, n (%) | 46 (12) | 18 (9) | 28 (14) | 0.117 | 42 (12) | 4 (7) | 0.230 |
| NT-proBNP, pg/ml (n); median (IQR) | 3042 (1313/8473) * | 8447 (5195/15,330) * | 1313 (579/2116) ‡ | <0.001 | 2927 (1282/8524) | 4280 (1624/8533) | 0.392 |
| Total cholesterol, mg/dl; median (IQR) | 175 (144/211) † | 167 (140/206) | 181 (150/217) | 0.003 | 175 (144/213) | 175 (142/205) | 0.564 |
| HDL, mg/dl; median (IQR) | 42 (34/49) | 39 (33/48) | 43 (34/51) | 0.052 | 42 (35/50) | 40 (32/49) | 0.438 |
| LDL, mg/dl; median (IQR) | 93 (72/116) | 92 (72/116) | 96 (71/116) | 0.814 | 89 (69/113) | 109 (91/168) | 0.076 |
| Creatine, mg/dl; median (IQR) | 1.13 (0.94/1.47) | 1.28 (1.00/1.74) | 1.06 (0.91/1.24) | <0.001 | 1.12 (0.94/1.46) | 1.18 (0.93/1.47) | 0.416 |
| eGFR, median (IQR) | 68 (49/93) * | 55 (35/80) ‡ | 81 (61/102) † | <0.001 | 66 (48/94) | 70 (51/89) | 0.432 |
| Sodium, mmol/L; median (IQR) | 139 (137/140) | 138 (136/141) | 139 (137/140) | 0.945 | 139 (137/141) | 138 (137/140) | 0.974 |
| Potassium, mmol/L; median (IQR) | 4.1 (3.8/4.3) | 4.1 (3.7/4.4) | 4.1 (3.8/4.3) | 0.679 | 4.1 (3.8/4.4) | 4.1 (3.9/4.3) | 0.264 |
| CRP, mg/dl; median (IQR) | 1.35 (0.48/4.77) | 2.43 (0.98/9.21) | 0.72 (0.35/2.17) | <0.001 | 1.41 (0.45/4.79) | 1.31 (0.54/3.65) | 0.706 |
| D-Dimer, µg/ml; median (IQR) | 1.03 (0.45/1.95) | 1.40 (0.86/2.49) | 0.54 (0.34/1.11) * | 0.004 | 0.95 (0.41/1.83) | 1.69 (0.86/2.61) | 0.200 |
| Fibrinogen mg/dl; median (IQR) | 482 (396/579) * | 525 (440/642) | 451 (376/537) | <0.001 | 481 (394/580) | 517 (424/578) | 0.430 |
| Prothrombine time, %; median (IQR) | 90 (75/107) | 87 (72/104) | 93 (78/108) | 0.012 | 90 (75/106) | 94 (78/112) | 0.253 |
| aPTT, s; median (IQR) | 37 (33/45) | 39 (34/46) | 36 (33/42) | 0.138 | 38 (34/44) | 37 (33/45) | 0.771 |
| INR; median (IQR) | 1.2 (1.1/1.4) | 1.4 (0.9/2.5) | 1.2 (1.1/1.3) | 0.052 | 1.2 (1.1/1.4) | 1.2 (1.1/1.3) | 0.988 |
| Heart rate, bpm; median (IQR) | 77 (66/88) | 80 (69/90) | 74 (64/84) | <0.001 | 77 (66/88) | 73 (66/86) | 0.999 |
| Rhythm | ‡ | 0.016 | 0.641 | ||||
| Sinus rhythm; n (%) | 345 (87) | 162 (81) | 183 (92) | - | 292 (86) | 53 (91) | - |
| Atrial fibrilation; n (%) | 35 (9) | 24 (12) | 11 (6) | - | 34 (9) | 4 (7) | - |
| Other; n (%) | 18 (4) | 13 (7) | 5 (2) | - | 17 (5) | 1 (2) | - |
| Left ventricular function, systolic | * | 0.017 | 0.983 | ||||
| Moderately impaired; n (%) | 148 (37) | 61 (31) | 87 (44) | - | 127 (37) | 21 (36) | - |
| Moderately to severley impaired; n (%) | 130 (33) | 68 (34) | 62 (31) | - | 111 (33) | 19 (33) | - |
| Severely impaired; n (%) | 120 (30) | 70 (35) | 50 (25) | - | 102 (30) | 18 (33) | - |
| No. of coronary vessels diseased | * | ‡ | 0.031 | <0.001 | |||
| 1 VD; n (%) | 112 (28) | 46 (23) | 66 (33) | - | 112 (33) | - | - |
| 2 VD; n (%) | 83 (21) | 39 (20) | 44 (22) | - | 72 (21) | 11 (19) | - |
| 3 VD; n (%) | 203 (51) | 114 (57) | 89 (45) | - | 156 (46) | 47 (81) | - |
| Revascularization procedure | ‡ | 0.011 | |||||
| PCI; n (%) | 340 (85) | 161 (81) | 179 (90) | ||||
| CABG; n (%) | 58 (15) | 38 (19) | 20 (10) | ||||
| All | All n = 398 | CABG n = 58 | PCI-All n = 340 | p-Value CABG vs. PCI-All |
|---|---|---|---|---|
| Total death; n (%) | 173 (44) | 21 (36) | 152 (45) | 0.227 |
| - Sudden death; n (%) | 16 (4) | 2 (3) | 14 (4) | - |
| - Heart failure; n (%) | 99 (25) | 14 (24) | 85 (25) | - |
| - Myocardial infarction; n (%) | 30 (8) | 1 (2) | 29 (9) | - |
| - Other; n (%) | 28 (7) | 4 (7) | 24 (7) | - |
| Alive; n (%) | 225 (56) | 37 (64) | 188 (55) | - |
| Above NT-pBNP median | All n = 199 | CABG n = 38 | PCI-All n = 161 | |
| Total death; n (%) | 123 (62) | 16 (45) | 107 (66) | 0.005 |
| - Sudden death; n (%) | 14 (7) | 2 (5) | 12 (8) | - |
| - Heart failure; n (%) | 70 (36) | 10 (26) | 60 (37) | - |
| - Myocardial infarction; n (%) | 21 (10) | 1 (3) | 20 (12) | - |
| - Other; n (%) | 18 (9) | 3 (8) | 15 (9) | - |
| Alive; n (%) | 76 (38) | 22 (58) | 54 (34) | - |
| Below NT-pBNP median | All n = 199 | CABG n = 20 | PCI-All n = 179 | |
| Total death; n (%) | 50 (25) | 5 (25) | 45 (25) | 0.989 |
| - Sudden death; n (%) | 2 (1) | 0 (0) | 2 (1) | - |
| - Heart failure; n (%) | 29 (15) | 4 (20) | 25 (14) | - |
| - Myocardial infarction; n (%) | 9 (5) | 0 (0) | 9 (5) | - |
| - Other; n (%) | 10 (5) | 1 (5) | 9 (5) | - |
| Alive; n (%) | 149 (75) | 15 (75) | 134 (75) | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, C.; Schneider, M.; Dalos, D.; Gangl, C.; Toth, C.; Goliasch, G.; Berger, R. High N-Terminal proB-Type Natriuretic Peptide Indicates Elevated Risk of Death after Percutaneous Coronary Intervention Compared to Coronary Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. J. Clin. Med. 2019, 8, 898. https://doi.org/10.3390/jcm8060898
Roth C, Schneider M, Dalos D, Gangl C, Toth C, Goliasch G, Berger R. High N-Terminal proB-Type Natriuretic Peptide Indicates Elevated Risk of Death after Percutaneous Coronary Intervention Compared to Coronary Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. Journal of Clinical Medicine. 2019; 8(6):898. https://doi.org/10.3390/jcm8060898
Chicago/Turabian StyleRoth, Christian, Matthias Schneider, Daniel Dalos, Clemens Gangl, Christian Toth, Georg Goliasch, and Rudolf Berger. 2019. "High N-Terminal proB-Type Natriuretic Peptide Indicates Elevated Risk of Death after Percutaneous Coronary Intervention Compared to Coronary Artery Bypass Surgery in Patients with Left Ventricular Dysfunction" Journal of Clinical Medicine 8, no. 6: 898. https://doi.org/10.3390/jcm8060898
APA StyleRoth, C., Schneider, M., Dalos, D., Gangl, C., Toth, C., Goliasch, G., & Berger, R. (2019). High N-Terminal proB-Type Natriuretic Peptide Indicates Elevated Risk of Death after Percutaneous Coronary Intervention Compared to Coronary Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. Journal of Clinical Medicine, 8(6), 898. https://doi.org/10.3390/jcm8060898