A Deep Brain Stimulation Trial Period for Treating Chronic Pain
Abstract
:1. Introduction
2. Anatomical Targets/Considerations
3. DBS Trial Period Safety and Risks
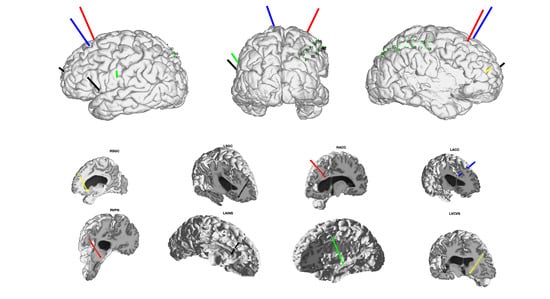
4. Motivation for a StereoEEG Trial in Patients with Chronic Pain
5. Detecting Biomarkers of Chronic Pain and Stimulation-Related Pain Relief
5.1. Biomarkers of Chronic Pain and Network Discovery
5.2. Biomarkers of Stimulation-Induced Pain Relief
6. Practical Considerations for Chronic Pain DBS Trial Period
Author Contributions
Funding
Conflicts of Interest
References
- Ben-Haim, S.; Mirzadeh, Z.; Rosenberg, W.S. Deep brain stimulation for intractable neuropathic facial pain. Neurosurg. Focus 2018, 45, E15. [Google Scholar] [CrossRef]
- Boccard, S.G.J.; Pereira, E.A.C.; Moir, L.; Aziz, T.Z.; Green, A.L. Long-term Outcomes of Deep Brain Stimulation for Neuropathic Pain. Neurosurgery 2013, 72, 221–231. [Google Scholar] [CrossRef]
- Levy, R.M.; Lamb, S.; Adams, J.E. Treatment of chronic pain by deep brain stimulation: Long term follow-up and review of the literature. Neurosurgery 1987, 21, 885–893. [Google Scholar] [CrossRef]
- Huang, K.T.; Martin, J.; Marky, A.; Chagoya, G.; Hatef, J.; Hazzard, M.A.; Thomas, S.M.; Lokhnygina, Y.; Lad, S.P. A National Survey of Spinal Cord Stimulation Trial-to-Permanent Conversion Rates. Neuromodul. Technol. Neural Interface 2015, 18, 133–140. [Google Scholar] [CrossRef]
- Jackson, T.; Thomas, S.; Stabile, V.; Shotwell, M.; Han, X.; McQueen, K. A Systematic Review and Meta-Analysis of the Global Burden of Chronic Pain without Clear Etiology in Low- and Middle-Income Countries: Trends in Heterogeneous Data and a Proposal for New Assessment Methods. Anesth. Analg. 2016, 123, 739–748. [Google Scholar] [CrossRef]
- Phillips, C.J. The Cost and Burden of Chronic Pain. Rev. Pain 2009, 3, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Farrell, S.; Green, A.; Aziz, T. The Current State of Deep Brain Stimulation for Chronic Pain and Its Context in Other Forms of Neuromodulation. Brain Sci. 2018, 8, 158. [Google Scholar] [CrossRef] [Green Version]
- Pool, J.L. Psychosurgery in Older People. J. Am. Geriatr. Soc. 1954, 2, 456–466. [Google Scholar] [CrossRef]
- Mazars, G.; Roge, R.; Mazars, Y. Results of the stimulation of the spinothalamic fasciculus and their bearing on the physiopathology of pain. Rev. Neurol. 1960, 103, 136–138. [Google Scholar]
- Hosobuchi, Y.; Adams, J.E.; Rutkin, B. Chronic Thalamic Stimulation for the Control of Facial Anesthesia Dolorosa. Arch. Neurol. 1973, 29, 158–161. [Google Scholar] [CrossRef]
- Nandi, D.; Yianni, J.; Humphreys, J.; Wang, S.; O’sullivan, V.; Shepstone, B.; Stein, J.F.; Aziz, T.Z. Phantom limb pain relieved with different modalities of central nervous system stimulation: A clinical and functional imaging case report of two patients. Neuromodul. J. Int. Neuromodul. Soc. 2004, 7, 176–183. [Google Scholar] [CrossRef]
- Adams, J.E. Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 1976, 2, 161–166. [Google Scholar] [CrossRef]
- Young, R.F.; Chambi, V.I. Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter: Evidence for a non-opioid mechanism. J. Neurosurg. 1987, 66, 364–371. [Google Scholar] [CrossRef]
- Coffey, R.J. Deep brain stimulation for chronic pain: Results of two multicenter trials and a structured review. Pain Med. Malden Mass 2001, 2, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Maslen, H.; Cheeran, B.; Pugh, J.; Pycroft, L.; Boccard, S.; Prangnell, S.; Green, A.L.; FitzGerald, J.; Savulescu, J.; Aziz, T. Unexpected Complications of Novel Deep Brain Stimulation Treatments: Ethical Issues and Clinical Recommendations. Neuromodul. Technol. Neural Interface 2018, 21, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijanki, K.R.; Manns, J.R.; Inman, C.S.; Choi, K.S.; Harati, S.; Pedersen, N.P.; Drane, D.L.; Waters, A.C.; Fasano, R.E.; Mayberg, H.S.; et al. Cingulum stimulation enhances positive affect and anxiolysis to facilitate awake craniotomy. J. Clin. Investig. 2019, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spisak, T.; Kincses, B.; Schlitt, F.; Zunhammer, M.; Schmidt-Wilcke, T.; Kincses, Z.T.; Bingel, U. Pain-free resting-state functional brain connectivity predicts individual pain sensitivity. Nat. Commun. 2020, 11, 187. [Google Scholar] [CrossRef] [Green Version]
- Lefaucheur, J.-P.; Drouot, X.; Cunin, P.; Bruckert, R.; Lepetit, H.; Créange, A.; Wolkenstein, P.; Maison, P.; Keravel, Y.; Nguyen, J.-P. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain 2009, 132, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Smith, H.; Owen, S.; Joint, C.; Stein, J.; Aziz, T. Peri-ventricular grey stimulation versus motor cortex stimulation for post stroke neuropathic pain. J. Clin. Neurosci. 2002, 9, 557–561. [Google Scholar] [CrossRef]
- Fontaine, D.; Hamani, C.; Lozano, A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: Critical review of the literature: Clinical article. J. Neurosurg. 2009, 110, 251–256. [Google Scholar] [CrossRef]
- Nguyen, J.-P.; Velasco, F.; Brugières, P.; Velasco, M.; Keravel, Y.; Boleaga, B.; Brito, F.; Lefaucheur, J.-P. Treatment of chronic neuropathic pain by motor cortex stimulation: Results of a bicentric controlled crossover trial. Brain Stimulat. 2008, 1, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Denis, D.J.; Marouf, R.; Rainville, P.; Bouthillier, A.; Nguyen, D.K. Effects of insular stimulation on thermal nociception. Eur. J. Pain 2016, 20, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Lempka, S.F.; Malone, D.A.; Hu, B.; Baker, K.B.; Wyant, A.; Ozinga, J.G.; Plow, E.B.; Pandya, M.; Kubu, C.S.; Ford, P.J.; et al. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann. Neurol. 2017, 81, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Shirvalkar, P.; Veuthey, T.L.; Dawes, H.E.; Chang, E.F. Closed-Loop Deep Brain Stimulation for Refractory Chronic Pain. Front. Comput. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Krames, E.S.; Monis, S.; Poree, L.; Deer, T.; Levy, R. Using the SAFE principles when evaluating electrical stimulation therapies for the pain of failed back surgery syndrome. Neuromodul. J. Int. Neuromodul. Soc. 2011, 14, 299–311, discussion 311. [Google Scholar] [CrossRef]
- Constantoyannis, C.; Berk, C.; Honey, C.R.; Mendez, I.; Brownstone, R.M. Reducing hardware-related complications of deep brain stimulation. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2005, 32, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.; Scelzo, E.; Locatelli, M.; Carrabba, G.; Levi, V.; Arlotti, M.; Barbieri, S.; Rampini, P.; Priori, A. Risk of Infection After Local Field Potential Recording from Externalized Deep Brain Stimulation Leads in Parkinson’s Disease. World Neurosurg. 2017, 97, 64–69. [Google Scholar] [CrossRef]
- Bojanic, S.; Sethi, H.; Hyam, J.; Yianni, J.; Nandi, D.; Joint, C.; Carter, H.; Gregory, R.; Bain, P.; Aziz, T.Z. Externalising deep brain electrodes: An increased risk of infection? J. Clin. Neurosci. 2004, 11, 732–734. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar] [CrossRef]
- Fields, H.L. How expectations influence pain. Pain 2018, 159 (Suppl. 1), S3–S10. [Google Scholar] [CrossRef] [Green Version]
- Beltrutti, D.; Lamberto, A.; Barolat, G.; Bruehl, S.P.; Doleys, D.; Krames, E.; Meglio, M.; North, R.; Olson, K.; Reig, E.; et al. The Psychological Assessment of Candidates for Spinal Cord Stimulation for Chronic Pain Management. Pain Pract. 2004, 4, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.N.; de Olivier, A.; Quesney, F.; Dubeau, F.; Savard, G.; Andermann, F. Efficacy of and morbidity associated with stereoelectroencephalography using computerized tomography—Or magnetic resonance imaging—Guided electrode implantation. J. Neurosurg. 2006, 104, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Ritzl, E.K.; Politsky, J.M.; Murro, A.M.; Smith, J.R.; Duckrow, R.B.; Spencer, D.D.; Bergey, G.K. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia 2004, 45, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.-W.; Kross, E. An fMRI-Based Neurologic Signature of Physical Pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, M.J.; Seymour, B. Decoding the matrix: Benefits and limitations of applying machine learning algorithms to pain neuroimaging. Pain 2014, 155, 864–867. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Baliki, M.N.; Farmer, M.A. Predicting transition to chronic pain. Curr. Opin. Neurol. 2013, 26, 360–367. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Burgess, R.C.; Malone, D.A.; Lempka, S.F.; Gale, J.T.; Floden, D.P.; Baker, K.B.; Machado, A.G. Deep brain stimulation of the ventral striatal area for poststroke pain syndrome: A magnetoencephalography study. J. Neurophysiol. 2018, 119, 2118–2128. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Jarry, G.; Drouot, X.; Ménard-Lefaucheur, I.; Keravel, Y.; Nguyen, J.-P. Motor cortex rTMS reduces acute pain provoked by laser stimulation in patients with chronic neuropathic pain. Clin. Neurophysiol. 2010, 121, 895–901. [Google Scholar] [CrossRef]
- Gross, J.; Schnitzler, A.; Timmerman, L.; Ploner, M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007, 5. [Google Scholar] [CrossRef] [Green Version]
- Bartolomei, F.; Lagarde, S.; Wendling, F.; McGonigal, A.; Jirsa, V.; Guye, M.; Benar, C. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia 2017, 58, 1131–1147. [Google Scholar] [CrossRef] [Green Version]
- Coghill, R.C.; McHaffie, J.G.; Yen, Y.-F. Neural correlates of interindividual differences in the subjective experience of pain. Proc. Natl. Acad. Sci. USA 2003, 100, 8538–8542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracey, I.; Woolf, C.J.; Andrews, N.A. Composite Pain Biomarker Signatures for Objective Assessment and Effective Treatment. Neuron 2019, 101, 783–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliki, M.N.; Chialvo, D.R.; Geha, P.Y.; Levy, R.M.; Harden, N.; Parrish, T.B.; Apkarian, A.V. Chronic Pain and the Emotional Brain: Specific Brain Activity Associated with Spontaneous Fluctuations of Intensity of Chronic Back Pain. J. Neurosci. 2006, 26, 12165–12173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apkarian, A.V.; Sosa, Y.; Sonty, S.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Gitelman, D.R. Chronic Back Pain Is Associated with Decreased Prefrontal and Thalamic Gray Matter Density. J. Neurosci. 2004, 24, 10410–10415. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.A.; Baliki, M.A.; Huang, L.; Baria, A.T.; Torbey, S.; Hermann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013, 136, 2751–2768. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.T.; Morrell, M.J. The RNS System: Responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev. Med. Devices 2014, 11, 563–572. [Google Scholar] [CrossRef]
- Medtronic FDA Approves Percept DBS Device. Available online: https://www.medtronic.com/us-en/about/news/fda-approval-percept.html (accessed on 20 September 2020).
- Ploner, M.; Gross, J.; Timmermann, L.; Pollok, B.; Schnitzler, A. Oscillatory activity reflects the excitability of the human somatosensory system. NeuroImage 2006, 32, 1231–1236. [Google Scholar] [CrossRef]
- Mouraux, A.; Guérit, J.M.; Plaghki, L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A partial partial differential- and C-fibre afferent volleys. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2003, 114, 710–722. [Google Scholar] [CrossRef]
- Babiloni, C.; Brancucci, A.; Del Percio, C.; Capotosto, P.; Arendt-Nielsen, L.; Chen, A.C.N.; Rossini, P.M. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J. Pain Off. J. Am. Pain Soc. 2006, 7, 709–717. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Hu, L.; Hung, Y.S.; Mouraux, A.; Iannetti, G.D. Gamma-Band Oscillations in the Primary Somatosensory Cortex—A Direct and Obligatory Correlate of Subjective Pain Intensity. J. Neurosci. 2012, 32, 7429–7438. [Google Scholar] [CrossRef] [Green Version]
- Chien, J.H.; Liu, C.C.; Kim, J.H.; Markman, T.M.; Lenz, F.A. Painful cutaneous laser stimuli induce event-related oscillatory EEG activities that are different from those induced by nonpainful electrical stimuli. J. Neurophysiol. 2014, 112, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.C.; Chien, J.H.; Kim, J.H.; Chuang, Y.F.; Cheng, D.T.; Anderson, W.; Lenz, F.A. Cross-frequency coupling in deep brain structures upon processing the painful sensory inputs. Neuroscience 2015, 303, 412–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Green, A.L.; Hyam, J.; Fitzgerald, J.; Aziz, T.Z.; Wang, S. Oscillatory neural representations in the sensory thalamus predict neuropathic pain relief by deep brain stimulation. Neurobiol. Dis. 2018, 109, 117–126. [Google Scholar] [CrossRef]
- Matsumoto, R.; Kunieda, T.; Nair, D. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure Eur. J. Epilepsy 2017, 44, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Mestre, T.A.; Lang, A.E.; Okun, M.S. Factors influencing the outcome of deep brain stimulation: Placebo, nocebo, lessebo, and lesion effects. Mov. Disord. 2016, 31, 290–298. [Google Scholar] [CrossRef]
- Hamani, C.; Schwalb, J.M.; Rezai, A.R.; Dostrovsky, J.O.; Davis, K.D.; Lozano, A.M. Deep brain stimulation for chronic neuropathic pain: Long-term outcome and the incidence of insertional effect. Pain 2006, 125, 188–196. [Google Scholar] [CrossRef]
- Foss, J.M.; Apkarian, A.V.; Chialvo, D.R. Dynamics of Pain: Fractal Dimension of Temporal Variability of Spontaneous Pain Differentiates Between Pain States. J. Neurophysiol. 2006, 95, 730–736. [Google Scholar] [CrossRef]
- Mercado, R.; Constantoyannis, C.; Mandat, T.; Kumar, A.; Schulzer, M.; Stoessl, A.J.; Honey, C.R. Expectation and the placebo effect in Parkinson’s disease patients with subthalamic nucleus deep brain stimulation. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 1457–1461. [Google Scholar] [CrossRef]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Cooper, S.E.; McIntyre, C.C.; Fernandez, H.H.; Vitek, J.L. Association of Deep Brain Stimulation Washout Effects with Parkinson Disease Duration. JAMA Neurol. 2013, 70, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, S.E.; Driesslein, K.G.; Noecker, A.M.; McIntyre, C.C.; Machado, A.M.; Butson, C.R. Anatomical targets associated with abrupt versus gradual washout of subthalamic deep brain stimulation effects on bradykinesia. PLoS ONE 2014, 9, e99663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, T.; Yohanandan, S.A.C.; Vogel, A.P.; McKay, C.M.; Jones, M.; Peppard, R.; McDermott, H.J. Deep brain stimulation wash-in and wash-out times for tremor and speech. Brain Stimul. Basic Transl. Clin. Res. Neuromodul. 2015, 8, 359. [Google Scholar] [CrossRef]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.D. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 2006, 10, 77–88. [Google Scholar] [CrossRef]
- Dualé, C.; Daveau, J.; Cardot, J.-M.; Boyer-Grand, A.; Schoeffler, P.; Dubray, C. Cutaneous Amitriptyline in Human VolunteersDifferential Effects on the Components of Sensory Information. Anesthesiol. J. Am. Soc. Anesthesiol. 2008, 108, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Andersson, G.; Haldrup, D. Personalized pain words and Stroop interference in chronic pain patients. Eur. J. Pain 2003, 7, 431–438. [Google Scholar] [CrossRef]
- Fichtenholtz, H.M.; Dean, H.L.; Dillon, D.G.; Yamasaki, H.; McCarthy, G.; LaBar, K.S. Emotion–attention network interactions during a visual oddball task. Cogn. Brain Res. 2004, 20, 67–80. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Reckziegel, D.; Vachon-Presseau, E.; Petre, B.; Schnitzer, T.J.; Baliki, M.; Apkarian, A.V. Deconstructing biomarkers for chronic pain: Context and hypothesis dependent biomarker types in relation to chronic pain. Pain 2019, 160, S37–S48. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Case, M.; Shirinpour, S.; He, B. Quantifying and Characterizing Tonic Thermal Pain Across Subjects From EEG Data Using Random Forest Models. IEEE Trans. Biomed. Eng. 2017, 64, 2988–2996. [Google Scholar] [CrossRef]
- Chiong, W.; Leonard, M.K.; Chang, E.F. Neurosurgical Patients as Human Research Subjects: Ethical Considerations in Intracranial Electrophysiology Research. Neurosurgery 2018, 83, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Andreassen Jaatun, E.A.; Hjermstad, M.J.; Gundersen, O.E.; Oldervoll, L.; Kaasa, S.; Haugen, D.F. Development and Testing of a Computerized Pain Body Map in Patients With Advanced Cancer. J. Pain Symptom Manag. 2014, 47, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wideman, T.H.; Edwards, R.R.; Walton, D.M.; Martel, M.O.; Hudon, A.; Seminowicz, D.A. The Multimodal Assessment Model of Pain. Clin. J. Pain 2019, 35, 212–221. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirvalkar, P.; Sellers, K.K.; Schmitgen, A.; Prosky, J.; Joseph, I.; Starr, P.A.; Chang, E.F. A Deep Brain Stimulation Trial Period for Treating Chronic Pain. J. Clin. Med. 2020, 9, 3155. https://doi.org/10.3390/jcm9103155
Shirvalkar P, Sellers KK, Schmitgen A, Prosky J, Joseph I, Starr PA, Chang EF. A Deep Brain Stimulation Trial Period for Treating Chronic Pain. Journal of Clinical Medicine. 2020; 9(10):3155. https://doi.org/10.3390/jcm9103155
Chicago/Turabian StyleShirvalkar, Prasad, Kristin K. Sellers, Ashlyn Schmitgen, Jordan Prosky, Isabella Joseph, Philip A. Starr, and Edward F. Chang. 2020. "A Deep Brain Stimulation Trial Period for Treating Chronic Pain" Journal of Clinical Medicine 9, no. 10: 3155. https://doi.org/10.3390/jcm9103155
APA StyleShirvalkar, P., Sellers, K. K., Schmitgen, A., Prosky, J., Joseph, I., Starr, P. A., & Chang, E. F. (2020). A Deep Brain Stimulation Trial Period for Treating Chronic Pain. Journal of Clinical Medicine, 9(10), 3155. https://doi.org/10.3390/jcm9103155