Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives
Abstract
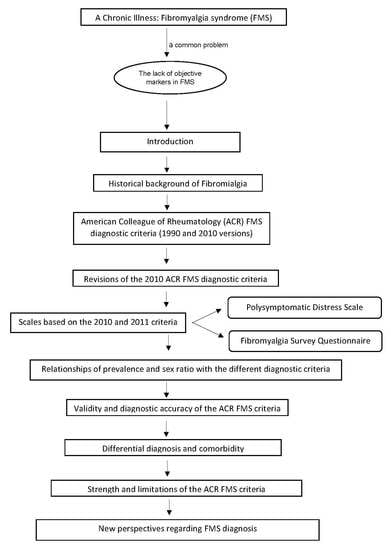
:1. Introduction
2. Historical Background on the Study of FMS
3. Development of the ACR FMS Diagnostic Criteria
4. Revisions of the 2010 ACR FMS Diagnostic Criteria
5. The Polysymptomatic Distress Scale and the FMS Survey Questionnaire
6. Associations of Prevalence and Sex Ratio with the Different Diagnostic Criteria
7. Validity of the ACR FMS Criteria
8. Differential Diagnosis and Comorbidity
9. Limitations of the ACR FMS Criteria
10. New Perspectives on FMS Diagnosis
11. Impact of the Novel Findings in the Diagnosis of FMS
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the classification of fibromyalgia. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoro, C.I.; Reyes del Paso, G.A. Personality and fibromyalgia: Relationships with clinical, emotional, and functional variables. Pers. Indiv. Differ. 2015, 85, 236–244. [Google Scholar] [CrossRef]
- Montoro, C.I.; Reyes del Paso, G.A.; Duschek, S. Alexithymia in Fibromyalgia Syndrome. Pers. Indiv. Differ. 2016, 102, 170–179. [Google Scholar] [CrossRef]
- Van Middendorp, H.; Lumley, M.A.; Jacobs, J.W.; van Doornen, L.J.; Bijlsma, J.W.; Geenen, R. Emotions and emotional approach and avoidance strategies in Fibromyalgia. J. Psychosom. Res. 2008, 64, 159–167. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Montoro, C.I.; Duschek, S.; Reyes del Paso, G.A. Depression and trait-anxiety mediate the influence of clinical pain on health-related quality of life in fibromyalgia. J. Affect. Disord. 2020, 265, 486–495. [Google Scholar] [CrossRef]
- Cabo-Meseguer, A.; Cerdá-Olmedo, G.; Trillo-Mata, J. Fibromialgia: Prevalencia, perfiles epidemiológicos y costes económicos. Med. Clin. 2017, 149, 441–448. [Google Scholar] [CrossRef]
- Sociedad Española de Reumatología-SER. Proyecto EPISER 2016: Prevalencia de las Enfermedades Reumáticas en la Población Adulta en España. 2016. Available online: https://www.ser.es/se-ha-presentado-el-estudio-episer-2016-en-la-sede-del-ministerio-de-sanidad-consumo-y-bienestar-social/ (accessed on 22 August 2019).
- Bohn, D.; Bernardy, K.; Wolfe, F.; Häuser, W. The association among childhood maltreatment, somatic symptom intensity, depression, and somatoform dissociative symptoms in patients with fibromyalgia syndrome: A single-center cohort study. J. Trauma Dissociation 2013, 14, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Kosseva, M.; Üceyler, N.; Klose, P.; Sommer, C. Emotional, physical, and sexual abuse in fibromyalgia syndrome: A systematic review with meta-analysis. Arthritis Care Res. 2011, 63, 808–820. [Google Scholar] [CrossRef]
- Goubert, D.; Danneels, L.; Graven-Nielsen, T.; Descheemaeker, F.; Meeus, M. Differences in pain processing between patients with chronic low back pain, recurrent low back pain, and fibromyalgia. Pain Physician 2017, 20, 307–338. [Google Scholar]
- Nijs, J.; Meeus, M.; Van Oosterwijck, J.; Roussel, N.; De Kooning, M.; Ickmans, K.; Matic, M. Treatment of central sensitization in patients with “unexplained” chronic pain: What options do we have? Expert Opin. Pharmacother. 2011, 12, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Koo, E.; Robinson, M.E.; Price, D.D. Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain 2007, 130, 177–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Coba, P.; Bruehl, S.; Moreno-Padilla, M.; Reyes Del Paso, G.A. Responses to slowly repeated evoked pain stimuli in fibromyalgia patients: Evidence of enhanced pain sensitization. Pain Med. 2017, 18, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- de la Coba, P.; Bruehl, S.; Galvez-Sánchez, C.M.; Reyes Del Paso, G.A. Specificity of slowly repeated evoked pain in comparison with traditional pain threshold and tolerance measures in fibromyalgia patients. Psychosom. Med. 2018, 80, 573–580. [Google Scholar]
- Gracely, R.H.; Petzke, F.; Wolf, J.M.; Clauw, D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002, 46, 1333–1343. [Google Scholar] [CrossRef]
- Montoro, C.I.; Duschek, S.; Muñoz Ladrón de Guevara, C.; Fernández-Serrano, M.J.; Reyes del Paso, G.A. Aberrant cerebral blood flow responses during cognition: Implications for the understanding of cognitive deficits in fibromyalgia. Neuropsychology 2015, 29, 173–182. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Garrido, S.; Pulgar, A.; Duschek, S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J. Psychosom. Res. 2011, 70, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Farhad, K.; Oaklander, A.L. Fibromyalgia and small-fiber polyneuropathy: what’s in a name? Muscle Nerve 2018, 58, 611–613. [Google Scholar] [CrossRef]
- Martínez-Lavín, M. Fibromyalgia and small fiber neuropathy: The plot thickens! Clin. Rheumatol. 2018, 37, 3167–3171. [Google Scholar] [CrossRef]
- Caro, X.J.; Galbraith, R.G.; Winter, E.F. Evidence of peripheral large nerve involvement in fibromyalgia: A retrospective review of EMG and nerve conduction findings in 55 FM subjects. Eur. J. Rheumatol. 2018, 5, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Hooten, W.M.; Timming, R.; Belgrade, M. Assessment and Management of Chronic Pain; Institute for Clinical Systems Improvement (ICSI): Bloomington, MN, USA, 2013. [Google Scholar]
- Moyano, S.; Kilstein, J.G.; de Miguel, C.A. Nuevos criterios diagnósticos de fibromialgia: ¿vinieron para quedarse? Reumatol. Clin. 2014, 3, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Okifuji, A.; Hare, B.D. Management of fibromyalgia syndrome: Review of evidence. Pain Ther. 2013, 2, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Baillou, G. Liber de Rheumatismo et Pleuritide Dorsali; Thevart MJ: Paris, France, 1642. [Google Scholar]
- Gowers, W.R. The development of the concept of fibrositis. Br. Med. J. 1904, 1, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Stockman, R. The causes, pathology, and treatment of chronic rheumatism. Edinb. Med. J. 1904, 15, 107–116. [Google Scholar]
- Vidal, L.F. Fibromialgia en la Práctica Diaria; Megatrazo S.A.C: Lima, Perú, 2015. [Google Scholar]
- Boland, E.W. Psychogenic rheumatism: The musculoskeletal expression of psychoneurosis. Ann. Rheum. Dis. 1947, 6, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, W. The fibrosits syndrome. Bull. Rheum. Dis. 1953, 8, 33–34. [Google Scholar]
- Traut, E.F. Fibrositis. J. Am. Geriatr. Soc. 1968, 16, 531–538. [Google Scholar] [CrossRef]
- Hench, P.K. Nonarticular rheumatism, 22nd rheumatism review: Review of the American and English literature for the years 1973 and 1974. Arthritis Rheumatol. 1976, 19, 1081–1089. [Google Scholar]
- Wolfe, F. Fibromyalgia wars. J. Rheumatol. 2009, 36, 671–678. [Google Scholar] [CrossRef]
- Block, S.R. Fibromyalgia and the rheumatisms. Common sense and sensibility. Rheum. Dis. Clin. North Am. 1993, 19, 61–78. [Google Scholar]
- Hadler, N.M. “Fibromyalgia” and the medicalization of misery. J. Rheumatol. 2003, 30, 1668–1670. [Google Scholar] [PubMed]
- Conrad, P. The Medicalization of Society; The John Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Hacking, I. The Social Construction of What? Harvard University Press: Boston, MA, USA, 1999. [Google Scholar]
- Conrad, P. The shifting engines of medicalization. J. Health Soc. Behav. 2005, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Smythe, H.A.; Moldofsky, H. Two contributions to understanding of the fibrositis syndrome. Bull. Rheum. Dis. 1977, 28, 928–931. [Google Scholar] [PubMed]
- Bennett, R.M. Fibrositis: Misnomer for a common rheumatic disorder. West J. Med. 1981, 134, 405–413. [Google Scholar]
- Hudson, J.I.; Hudson, M.S.; Pliner, L.F.; Goldenberg, D.L.; Pope, H.G., Jr. Fibromyalgia and major affective disorder: A controlled phenomenology and family history study. Am. J. Psychiatry 1985, 142, 441–446. [Google Scholar]
- Yunus, M.; Masi, A.T.; Calabro, J.J.; Miller, K.A.; Feigenbaum, S.L. Primary fibromyalgia (fibrositis): Clinical study of 50 patients with matched normal controls. Semin. Arthritis Rheum. 1981, 11, 151–171. [Google Scholar] [CrossRef]
- Payne, T.C.; Leavitt, F.; Garron, D.C.; Katz, R.S.; Golden, H.E.; Glickman, P.B.; Vanderplate, C. Fibrositis and psychologic disturbance. Arthritis Rheum. 1982, 25, 213–217. [Google Scholar] [CrossRef]
- Wolfe, F.; Cathey, M.A. Prevalence of primary and secondary fibrositis. J. Rheumatol. 1983, 10, 965–968. [Google Scholar]
- Campbell, S.M.; Clark, S.; Tindall, E.A.; Forehand, M.E.; Bennett, R.M. Clinical characteristics of fibrositis I. A “blinded” controlled study of symptoms and tender points. Arthritis Rheum. 1983, 26, 817–824. [Google Scholar] [CrossRef]
- Greenfield, S.; Fitzcharles, M.A.; Esdaile, J.M. Reactive fibromyalgia syndrome. Arthritis Rheumatol. 1992, 35, 678–681. [Google Scholar] [CrossRef]
- Raspe, H.; Baugmanter, C.; Wolfe, F. The prevalence of fibromyalgia in a rural German community: How much difference do different criteria make? Arthritis Rheumatol. 1993, 36, S48. [Google Scholar]
- Borenstein, D. Prevalence and treatment outcome of primary and secondary fibromyalgia in patients with spinal pain. Spine 1995, 20, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Heymann, R.E.; Paiva, E.S.; Martinez, J.E.; Helfenstein, M.; Rezendea, M.C.; Provenza, R.; Ranzolin, A.; Renato de Assis, A.; Feldman, D.P.; Severiano, L.; et al. Novas diretrizes para o diagnóstico da fibromyalgia. Rev. Bras. Reumatol. 2017, 57, S467–S476. [Google Scholar] [CrossRef]
- Wolfe, F.; Häuser, W. Review article Fibromyalgia diagnosis and diagnostic criteria. Ann. Med. 2011, 43, 495–502. [Google Scholar] [CrossRef]
- Okifuji, A.; Turk, D.C.; Sinclair, J.D.; Starz, T.W.; Marcus, D.A. A standardized manual tender point survey. I. Development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J. Rheumatol. 1997, 24, 377–383. [Google Scholar] [PubMed]
- Wolfe, F. The Status of Fibromyalgia Criteria. Arthritis Rheumatol. 2015, 67, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Bidari, A.; Hassanzadeh, M.; Ghavidel Parsa, B.; Kianmehr, N.; Kabir, A.; Pirhadi, S.; Sayfi, M.; Toutounchi, M.; Fattahi, F.; Zandi Karimi, F. Validation of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia in an Iranian population. Rheumatol. Int. 2013, 33, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Usui, C.; Hatta, K.; Aratani, S.; Yagishita, N.; Nishioka, K.; Kanazawa, T.; Ito, K.; Yamano, Y.; Nakamura, H.; Nakajima, T.; et al. The Japanese version of the 2010 American college of rheumatology preliminary diagnostic criteria for fibromyalgia and the fibromyalgia symptom scale: Reliability and validity. Mod. Rheumatol. 2012, 22, 40–44. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Ste-Marie, P.A.; Panopalis, P.; Ménard, H.; Shir, Y.; Wolfe, F. The 2010 American college of rheumatology fibromyalgia survey diagnostic criteria and symptom severity scale is a valid and reliable tool in a French speaking fibromyalgia cohort. BMC Musculoskelet. Disord. 2012, 13, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanmaz, M.N.; Sevgi, A.; Mualla, B. The reliability and validity of the Turkish version of fibromyalgia survey diagnostic criteria and symptom severity scale. J. Back Musculoskelet. Rehabil. 2016, 29, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, B.; García-Fructuoso, F.; Belenguer, R.; Alegre, C.; Moreno-Muelas, J.V.; Hernández, J.L.; Pina, T.; González-Gay, M.Á. The Spanish version of the 2010 American College of Rheumatology Preliminary Clinical Diagnostic Criteria for fibromyalgia: Reliability and validity assessment. Exp. Rheumatol. 2016, 34, S55–S58. [Google Scholar]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Bovee, C.E.; Robinson, M.E.; Price, D.D. Cutaneous C fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain 2008, 139, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderschueren, S.; Van Wambeke, P.; Morlion, B. Fibromyalgia: Do not give up the tender point count too easily: Comment on the article by Wolfe. Arthritis Care Res. 2010, 62, 1676–1678. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. Reply [letter]. Arthritis Care Res. 2011, 63, 309–310. [Google Scholar] [CrossRef] [Green Version]
- Segura-Jiménez, V.; Aparicio, V.A.; Álvarez, I.C.; Estévez-López, F.; Delgado-Fernández, M.; Carbonell-Baeza, A. Validation of the modified 2010 American College of Rheumatology diagnostic criteria for fibromyalgia in a Spanish population. Rheumatology 2014, 53, 1803–1811. [Google Scholar] [CrossRef] [Green Version]
- Usui, C.; Hatta, K.; Aratani, S.; Yagishita, N.; Nishioka, K.; Kanazawa, T.; Itoh, K.; Yamano, Y.; Nakamura, H.; Nakajima, T.; et al. The Japanese version of the modified ACR Preliminary Diagnostic Criteria for Fibromyalgia and the Fibromyalgia Symptom Scale: Reliability and validity. Mod. Rheumatol. 2013, 23, 846–850. [Google Scholar] [CrossRef]
- Ahmed, S.; Aggarwal, A.; Lawrence, A. Performance of the American College of Rheumatology 2016 criteria for fibromyalgia in a referral care setting. Rheumatol. Int. 2019, 39, 1397–1403. [Google Scholar] [CrossRef]
- Littlejohn, G.O.; Guymer, E.K. In clinical practice, the term “central sensitivity score” is more useful than the term “polysymptomatic distress scale”: Comment on the editorial by Wolfe. Arthritis Rheumatol. 2015, 67, 2553. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Wallit, B.; Rasker, J.J.; Katz, R.S.; Häuser, W. The use of polysymptomatic distress categories in the evaluation of fibromyalgia (FM) and FM severity. J. Rheumatol. 2015, 42, 1494–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-de-la-Peña, M.T.; Triñanes, Y.; González-Villar, A.; Romero-Yuste, S.; Gómez-Perretta, C.; Arias, M.; Wolfe, F. Convergence between the 1990 and 2010 ACR diagnostic criteria and validation of the Spanish version of the Fibromyalgia Survey Questionnaire (FSQ). Rheumatol. Int. 2015, 35, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Jung, E.; Erbslöh-Möller, B.; Gesmann, M.; Kühn-Becker, H.; Petermann, F.; Langhorst, J.; Weiss, T.; Winkelmann, A.; Wolfe, F. Validation of the Fibromyalgia Survey Questionnaire within a cross-sectional survey. PLoS ONE 2012, 7, e37504. [Google Scholar] [CrossRef] [PubMed]
- Bidari, A.; Ghavidel Parsa, B.; Amir Maafi, A.; Montazeri, A.; Ghalehbaghi, B.; Hassankhani, A.; Aarabi, Y.; Haghdoost, A. Validation of fibromyalgia survey questionnaire and polysymptomatic distress scale in a Persian population. Rheumatol. Int. 2015, 35, 2013–2019. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Soriano-Maldonado, A.; Álvarez-Gallardo, I.C.; Estévez-López, F.; Carbonell-Baeza, A.; Delgado-Fernández, M. Subgroups of fibromyalgia patients using the 1990 American College of Rheumatology criteria and the modified 2010 preliminary diagnostic criteria: The al-Ándalus project. Clin. Exp. Rheumatol. 2016, 34, S26–S33. [Google Scholar]
- Galvez-Sánchez, C.M.; de la Coba, P.; Duschek, S.; Reyes del Paso, G.A. Reliability, factor structure and predictive validity of the Widespread Pain Index and Symptom Severity scales of the 2010 American College of Rheumatology criteria of fibromyalgia. Pain Med. 2020. Under review. [Google Scholar]
- Estévez-López, F.; Segura-Jiménez, V.; Álvarez-Gallardo, I.C.; Borges-Cosic, M.; Pulido-Martos, M.; Carbonell-Baeza, A.; Aparicio, V.A.; Geenen, R.; Delgado-Fernández, M. Adaptation profiles comprising objective and subjective measures in fibromyalgia: The al-Ándalus project. Rheumatology 2017, 56, 2015–2024. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.; Pennebaker, J.W. Health complaints, stress and distress: Exploring the central role of negative affectivity. Psychol. Rev. 1989, 96, 234–254. [Google Scholar] [CrossRef]
- Finan, P.H.; Zautra, A.J.; Davis, M.C. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom. Med. 2009, 71, 474–482. [Google Scholar] [CrossRef]
- Gracely, R.H.; Grant, M.A.; Giesecke, T. Evoked pain measures in fibromyalgia. Best Pract. Res. Clin. Rheumatol. 2003, 17, 593–609. [Google Scholar] [CrossRef]
- Jones, G.T.; Atzeni, F.; Beasley, M.; Flüß, E.; Sarzi-Puttini, P.; Macfarlane, G.J. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol 2016, 7, 568–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, F.; Walitt, B.; Perrot, S.; Rasker, J.J.; Häuser, W. Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS ONE 2018, 13, e0203755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Maloney, E.; Wright, B.; Kennedy, M.; Kallail, K.J.; Rasker, J.J.; Häuser, W.; Wolfe, F. The problematic nature of Fibromyalgia diagnosis in the community. ACR Open Rheumatol. 2019, 1, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Toda, K. Preliminary diagnostic criteria for fibromyalgia should be partially revised: Comment on the article by Wolfe et al. Arthritis Care Res. 2011, 63, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Sarzi-Puttini, P.; Fitzcharles, M.A. Fibromyalgia syndrome: Under-, over- and misdiagnosis. Clin. Exp. Rheumatol. 2019, 37, 90–97. [Google Scholar]
- Walitt, B.; Nahin, R.L.; Katz, R.S.; Bergman, M.J.; Wolfe, F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS ONE 2015, 10, e0138024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walitt, B.; Katz, R.S.; Bergman, M.J.; Wolfe, F. Three-quarters of persons in the US population reporting a clinical diagnosis of fibromyalgia do not satisfy fibromyalgia criteria: The 2012 National Health Interview Survey. PLoS ONE 2016, 11, e0157235. [Google Scholar] [CrossRef] [Green Version]
- Doran, E.; Henry, D. Disease mongering: Expanding the boundaries of treatable disease. Intern. Med. J. 2008, 38, 858–861. [Google Scholar] [CrossRef]
- Tikkinen, K.A.; Leinonen, J.S.; Guyatt, G.H.; Ebrahim, S.; Järvinen, T. What is a disease? Perspectives of the public, health professionals and legislators. BMJ Open 2012, 2, e001632. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Schmukler, J.; Shakeel, J.; Castrejon, I.; Gibson, K.A.; Srinivasan, S.; Häuser, W.; Pincus, T. Diagnosis of Fibromyalgia: Disagreement between Fibromyalgia Criteria and Clinician-Based Fibromyalgia Diagnosis in a University Clinic. Artritis Care Res. 2019, 71, 343–351. [Google Scholar] [CrossRef]
- Kumbhare, D.; Ahmed, S.; Sander, T.; Grosman-Rimon, L.; Srbely, J. A survey of physicians’ knowledge and adherence to the diagnostic criteria for fibromyalgia. Pain Med. 2018, 9, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, S.; Lundman, B. Transitions experienced by women with fibromyalgia. Health Care Women Int. 2001, 22, 617–631. [Google Scholar] [CrossRef]
- Kool, M.B.; Van de Schoot, R.; López-Chicheri, I.; Mewes, R.; Da Silva, J.A.; Vangronsveld, K.; Wismeijer, A.A.; Lumley, M.A.; van Middendorp, H.; Bijlsma, J.W.; et al. Measurement invariance of the Illness Invalidation Inventory (3*I) across language, rheumatic disease and gender. Ann. Rheum. Dis. 2014, 73, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kool, M.B.; van de Schoot, R.; Boeije, H.R.; Geenen, R. Understanding the lack of understanding: Invalidation from the perspective of the patient with fibromyalgia. Arthritis Rheum. 2009, 61, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J. AAPT Diagnostic Criteria for Fibromyalgia. J. Pain 2019, 20, 611–628. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J. Response to Wolfe. Letter to the Editor, “Fibromyalgia Criteria”. J. Pain 2019, 20, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F. Letter to the editor, “Fibromyalgia Criteria”. J. Pain 2019, 20, 739–740. [Google Scholar] [CrossRef]
- de la Coba, P.; Bruehl, S.; Reyes del Paso, G.A. Addition of slowly repeated evoked pain responses to clinical symptoms enhances fibromyalgia diagnostic accuracy. Pain Med. 2019. [Google Scholar] [CrossRef]




| Authors | Required Tender Points |
|---|---|
| Smythe & Moldofsky [39] | 12 of 14 |
| Bennett et al. [40] | 10 of 25 |
| Yunus et al. [42] | 3–5 of 40 |
| Payne et al. [43] | 4 of 14 |
| Wolfe & Cathey [44] | 7 of 14 |
| Campbell et al. [45] | 12 of 17 |
| Wolfe et al. [1] | 11 of 18 |
| Greenfield et al. [46] | ≥7 |
| Raspe et al. [47] | ≥17 tender points and ≤2 control tender points |
| Borenstein [48] | 11 of 18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Sánchez, C.M.; Reyes del Paso, G.A. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J. Clin. Med. 2020, 9, 1219. https://doi.org/10.3390/jcm9041219
Galvez-Sánchez CM, Reyes del Paso GA. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. Journal of Clinical Medicine. 2020; 9(4):1219. https://doi.org/10.3390/jcm9041219
Chicago/Turabian StyleGalvez-Sánchez, Carmen M., and Gustavo A. Reyes del Paso. 2020. "Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives" Journal of Clinical Medicine 9, no. 4: 1219. https://doi.org/10.3390/jcm9041219
APA StyleGalvez-Sánchez, C. M., & Reyes del Paso, G. A. (2020). Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. Journal of Clinical Medicine, 9(4), 1219. https://doi.org/10.3390/jcm9041219