Estimation of Starch Hydrolysis in Sweet Potato (Beni Haruka) Based on Storage Period Using Nondestructive Near-Infrared Spectrometry
Abstract
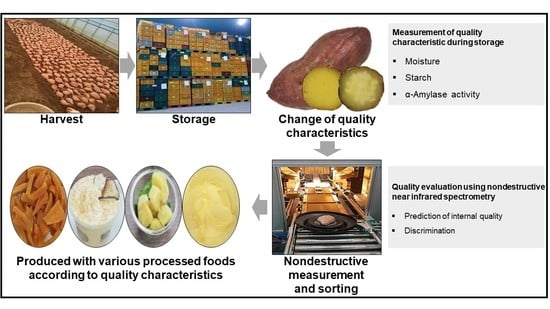
:1. Introduction
2. Materials and Methods
2.1. Sweet Potatoes
2.2. Measurement of Quality Characteristics During Storage
2.2.1. Moisture
2.2.2. Starch
2.2.3. α-Amylase Assay
2.3. Quality Evaluation Using Nondestructive Techniques
2.3.1. Measurement of NIR Spectrum
2.3.2. Chemometrics
Pretreatment of NIR Spectrum
Prediction of Post-harvest Quality Using NIR
Discrimination of Sweet Potatoes Using NIR
3. Results
3.1. Measurement of Quality Characteristics During Storage
3.2. Quality Evaluation Using Nondestructive Techniques
3.2.1. Spectral Characteristics of NIR Acquisition
3.2.2. Prediction of Internal Quality Using NIR
3.2.3. Discrimination of Sweet Potatoes by NIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanraj, R.; Sivasankar, S. Sweet Potato (Ipomoea batatas [L.] Lam)-A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef]
- Antial, B.S.; Akpanz, E.J.; Okonl, P.A.; Umorenl, I.U. Nutritive and anti-nutritive evaluation of sweet potatoes. Pak. J. Nutr. 2006, 5, 166–168. [Google Scholar] [CrossRef]
- Cartier, A.; Woods, J.; Sismour, E.; Allen, J.; Ford, E.; Githinji, L.; Xu, Y. Physiochemical, nutritional and antioxidant proper-ties of fourteen Virginia-grown sweet potato varieties. J. Food Meas. Charact. 2017, 11, 1333–1341. [Google Scholar] [CrossRef]
- Truong, V.D.; Avula, R.Y.; Pecota, K.V.; Yencho, G.C. Sweetpotato production, processing, and nutritional quality. In Handbook of Vegetables and Vegetable Processing, 2nd ed.; Siddiq, M., Uebersax, M.A., Eds.; Wiley: New York, NY, USA, 2018; Volume 2, pp. 811–838. [Google Scholar]
- Bovell-Benjamin, A.C. Sweet Potato: A Review of its Past, Present, and Future Role in Human Nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar] [CrossRef]
- Zhang, H.; Patel, J.; Bhunia, K.; Al-Ghamdi, S.; Sonar, C.R.; Ross, C.F.; Tang, J.; Sablani, S. Color, vitamin C, β-carotene and sensory quality retention in microwave-assisted thermally sterilized sweet potato puree: Effects of polymeric package gas barrier during storage. Food Packag. Shelf Life 2019, 21, 100324. [Google Scholar] [CrossRef]
- Nath, P.; Kale, S.J.; Chauhan, O.P.; Gupta, R.K. High Pressure Processing Induced Changes in Bioactive Compounds, Antioxidant Activity, Microbial Safety and Color Attributes of Coriander Paste. Agric. Res. 2016, 5, 182–192. [Google Scholar] [CrossRef]
- Renu, R.; Chidanand, D.V. Internal quality classification of agricultural produce using non-destructive image processing technologies (soft X-ray). Int. J. Latest Trends Eng. Technol. 2013, 2, 535–543. [Google Scholar]
- Kushman, L.J.; Wright, F.S. Sweetpotato storage. In Agriculture Handbook; Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1969; Volume 358, pp. 1–12. [Google Scholar]
- Jeong, B.C.; Ahn, Y.S.; Chung, M.N.; Lee, J.S.; Oh, Y.H. Current status and prospect of quality evaluation in sweetpotato. Korean J. Crop Sci. 2002, 47, 124–134. [Google Scholar]
- Tomlins, K.; Ndunguru, G.; Rwiza, E.; Westby, J. Influence of pre-harvest curing and mechanical injury on the quality and shelf-life of sweet potato (Ipomoea batatas (L.) Lam) in East Africa. J. Hortic. Sci. Biotechnol. 2002, 77, 399–403. [Google Scholar] [CrossRef]
- Nabubuya, A.; Namutebi, A.; Byaruhanga, Y.; Narvhus, J.; Wicklund, T. Influence of development, postharvest handling, and storage conditions on the carbohydrate components of sweetpotato (Ipomea batatas Lam.) roots. Food Sci. Nutr. 2017, 5, 1088–1097. [Google Scholar] [CrossRef] [Green Version]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Crop sensor based non-destructive estimation of nitrogen nutritional status, yield, and grain protein content in wheat. Agriculture 2020, 10, 148. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Cozzolino, D.; Porker, K.; Laws, M. An Overview on the Use of Infrared Sensors for in Field, Proximal and at Harvest Monitoring of Cereal Crops. Agriculture 2015, 5, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Wang, A.; Xu, H.; Fu, X.; Ying, Y. Applications of Near-Infrared Systems for Quality Evaluation of Fruits: A Review. Trans. ASABE 2016, 59, 399–419. [Google Scholar] [CrossRef]
- Katayama, K.; Komaki, K.; Tamiya, S. Prediction of starch, moisture, and sugar in sweet potato by near infrared transmittance. HortScience 1996, 31, 1003–1006. [Google Scholar] [CrossRef] [Green Version]
- Iwase, J.; Sato, Y.; Comparini, D.; Masi, E.; Mancuso, S.; Kawano, T. Non-invasive acoustic sensing of tuberous roots of sweet potato (Ipomoea batatas) growing belowground. Hortscience 2015, 29, 176–180. [Google Scholar]
- Chen, J.Y.; Zhang, H.; Miao, Y.; Asakura, M. Nondestructive Determination of Sugar Content in Potato Tubers Using Visible and Near Infrared Spectroscopy. Jpn. J. Food Eng. 2010, 11, 59–64. [Google Scholar] [CrossRef]
- Camps, C.; Camps, Z.-N. Optimized Prediction of Reducing Sugars and Dry Matter of Potato Frying by FT-NIR Spectroscopy on Peeled Tubers. Molculers 2019, 24, 967. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.-F.; Lv, C.-X.; Yuan, Y.-W.; Yang, B.-N.; Zhao, Q.-L.; Cao, Y.-F. Non-destructive Detection of Blackheart Potatoes Based on Energy Spectrum of VIS/NIR Transmittance. IOP Conf. Ser. Earth Environ. Sci. 2020, 512, 012060. [Google Scholar] [CrossRef]
- Rady, A.M.; Guyer, D.E. Rapid and/or nondestructive quality evaluation methods for potatoes: A review. Comput. Electron. Agric. 2015, 117, 31–48. [Google Scholar] [CrossRef]
- Rady, A.M.; Guyer, D.E. Evaluation of sugar content in potatoes using NIR reflectance and wavelength selection techniques. Postharvest Biol. Technol. 2015, 103, 17–26. [Google Scholar] [CrossRef]
- Roggo, Y.; Chalus, P.; Maurer, L.; Lema-Martinez, C.; Edmond, A.; Jent, N. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J. Pharm. Biomed. Anal. 2007, 44, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Emerging non-destructive thermal imaging technique coupled with chemometrics on quality and safety inspection in food and agriculture. Trends Food Sci. Technol. 2020, 105, 176–185. [Google Scholar] [CrossRef]
- Gislum, R.; Nikneshan, P.; Shrestha, S.; Tadayyon, A.; Deleuran, L.C.; Boelt, B. Characterisation of Castor (Ricinus communis L.) Seed Quality Using Fourier Transform Near-Infrared Spectroscopy in Combination with Multivariate Data Analysis. Agriculture 2018, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.Z.; Kvalheim, O.M. Robust methods for multivariate analysis—A tutorial review. Chemom. Intell. Lab. Syst. 1996, 32, 1–10. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; González-Casado, A.; Bagur-González, M.G.; Cuadros-Rodríguez, L. Alternative data min-ing/machine learning methods for the analytical evaluation of food quality and authenticity–A review. Food Res. Int. 2019, 122, 25–39. [Google Scholar] [CrossRef]
- Ai, J.; Witt, T.; Cowin, G.; Dhital, S.; Turner, M.S.; Stokes, J.R.; Gidley, M.J. Anti-staling of high-moisture starchy food: Effect of hydrocolloids, emulsifiers and enzymes on mechanics of steamed-rice cakes. Food Hydrocoll. 2018, 83, 454–464. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trac-Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Zimmermann, B.; Kohler, A. Optimizing Savitzky–Golay Parameters for Improving Spectral Resolution and Quantification in Infrared Spectroscopy. Appl. Spectrosc. 2013, 67, 892–902. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.J.; Xing, L.; Han, L.J. Influence of data preprocessing on the quantitative determination of nutrient content in poultry manure by near infrared spectroscopy. J. Environ. Qual. 2010, 39, 1841–1847. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- da Costa, N.L.; da Costa, M.S.; Barbosa, R. A Review on the application of chemometrics and machine learning algorithms to evaluate beer authentication. Food Anal. Meth. 2021, 14, 136–155. [Google Scholar] [CrossRef]
- Coûteaux, M.M.; Sarmiento, L.; Herve´, D.; Acevedo, D. Determination of water-soluble and total extractable polyphenolics in biomass, necromass and decomposing plant material using near-infrared reflectance spectroscopy (NIRS). Soil Biol. Biochem. 2005, 37, 795–799. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Santos, A.; Rozenberg, P.; Pâques, L.E.; Charpentier, J.P.; Schwanninger, M.; Rodrigues, J. A common near infra-red—based partial least squares regression model for the prediction of wood density of Pinus pinaster and Larix × eurolepis. Wood Sci. Technol. 2012, 46, 157–175. [Google Scholar] [CrossRef]
- D’Acqui, L.P.; Pucci, A.; Janik, L.J. Soil properties prediction of western Mediterranean islands with similar climatic environments by means of mid-infrared diffuse reflectance spectroscopy. Eur. J. Soil Sci. 2010, 61, 865–876. [Google Scholar] [CrossRef]
- Gad, H.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Al-Azizi, M.M. Application of Chemometrics in Authentication of Herbal Medicines: A Review. Phytochem. Anal. 2012, 24, 1–24. [Google Scholar] [CrossRef]
- Nabubuya, A.; Namutebi, A.; Byaruhanga, Y.B.; Narvhus, J.; Stenstrøm, Y.; Wicklund, T.; Nabubuya, A.; Yusuf, B.; Judith, N.; Yngve, S.; et al. Amylolytic Activity in Selected Sweetpotato (Ipomoea batatas Lam) Varieties during Development and in Storage. Food Nutr. Sci. 2012, 3, 660–668. [Google Scholar] [CrossRef] [Green Version]
- Hagenimana, V.; Vezina, L.P.; Simard, R.E. Distribution of amylases within sweet potato (Ipomoea batatas L.) root tissue. J. Agric. Food Chem. 1992, 40, 1777–1783. [Google Scholar] [CrossRef]
- Sarikaya, E.; Higasa, T.; Adachi, M.; Mikami, B. Comparison of degradation abilities of α-and β-amylases on raw starch granules. Process Biochem. 2000, 35, 711–715. [Google Scholar] [CrossRef]
- Luck, W.A.P. Infrared overtone region. In Structure of Water and Aqueous Solutions; Luck, W.A.P., Ed.; Weinheim Verlag Chemie: Weinheim, Germany, 1974; pp. 248–284. [Google Scholar]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Osborne, B.G. Near-infrared spectroscopy in food analysis. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, 1st ed.; Meyers, R.A., Ed.; Wiley: New York, NY, USA, 2006; Volume 1, pp. 1–14. [Google Scholar]
- Farhadi, R.; Afkari-Sayyah, A.H.; Jamshidi, B.; Gorji, A.M. Prediction of internal compositions change in potato during storage using visible/near-infrared (Vis/NIR) spectroscopy. Int. J. Food Eng. 2020, 16. [Google Scholar] [CrossRef]
- Lu, G.; Huang, H.; Zhang, D. Prediction of sweetpotato starch physiochemical quality and pasting properties using near-infrared reflectance spectroscopy. Food Chem. 2006, 94, 632–639. [Google Scholar] [CrossRef]
- Ding, X.; Ni, Y.; Kokot, S. NIR spectroscopy and chemometrics for the discrimination of pure, powdered, purple sweet po-tatoes and their samples adulterated with the white sweet potato flour. Chemom. Intell. Lab. Syst. 2015, 144, 17–23. [Google Scholar] [CrossRef]
- De Carvalho, L.C.; Morais, C.L.M.; De Lima, K.M.G.; Junior, L.C.C.; Nascimento, P.; De Faria, J.B.; Teixeira, G.H.D.A. Determination of the geographical origin and ethanol content of Brazilian sugarcane spirit using near-infrared spectroscopy coupled with discriminant analysis. Anal. Methods 2016, 8, 5658–5666. [Google Scholar] [CrossRef]







| Quality Parameters | LVs | Calibration | Cross-Validation | Prediction | RMSEP/ RMSECV | RPD | |||
|---|---|---|---|---|---|---|---|---|---|
| RC2 | RMSEC | RCV2 | RMSECV | RP2 | RMSEP | ||||
| Raw spectrum | |||||||||
| Moisture | 3 | 0.5893 | 1.5508 | 0.5698 | 1.6027 | 0.6533 | 1.6706 | 1.0424 | 1.3258 |
| Starch | 3 | 0.5825 | 16.6109 | 0.5538 | 17.3473 | 0.4607 | 17.9101 | 1.0324 | 1.0211 |
| Savitzky–Golay derivative | |||||||||
| Moisture | 7 | 0.7878 | 1.1147 | 0.6920 | 1.3520 | 0.8031 | 1.2590 | 0.9312 | 2.1655 |
| Starch | 4 | 0.7303 | 13.3509 | 0.6896 | 14.4122 | 0.6462 | 14.5069 | 1.0066 | 1.6605 |
| SNV | |||||||||
| Moisture | 8 | 0.7715 | 1.1568 | 0.6919 | 1.3551 | 0.8047 | 1.2539 | 0.9253 | 2.0036 |
| Starch | 9 | 0.8320 | 10.5383 | 0.7688 | 12.5890 | 0.7722 | 11.6418 | 0.9248 | 2.1288 |
| MSC | |||||||||
| Moisture | 7 | 0.7531 | 1.2024 | 0.7175 | 1.4021 | 0.8065 | 1.2480 | 0.8901 | 2.0136 |
| Starch | 8 | 0.7734 | 12.2385 | 0.6784 | 14.8012 | 0.7811 | 11.4115 | 0.7710 | 2.1749 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Choi, M.-H.; Shin, H.-J. Estimation of Starch Hydrolysis in Sweet Potato (Beni Haruka) Based on Storage Period Using Nondestructive Near-Infrared Spectrometry. Agriculture 2021, 11, 135. https://doi.org/10.3390/agriculture11020135
Kim D-S, Choi M-H, Shin H-J. Estimation of Starch Hydrolysis in Sweet Potato (Beni Haruka) Based on Storage Period Using Nondestructive Near-Infrared Spectrometry. Agriculture. 2021; 11(2):135. https://doi.org/10.3390/agriculture11020135
Chicago/Turabian StyleKim, Da-Song, Moon-Hee Choi, and Hyun-Jae Shin. 2021. "Estimation of Starch Hydrolysis in Sweet Potato (Beni Haruka) Based on Storage Period Using Nondestructive Near-Infrared Spectrometry" Agriculture 11, no. 2: 135. https://doi.org/10.3390/agriculture11020135
APA StyleKim, D. -S., Choi, M. -H., & Shin, H. -J. (2021). Estimation of Starch Hydrolysis in Sweet Potato (Beni Haruka) Based on Storage Period Using Nondestructive Near-Infrared Spectrometry. Agriculture, 11(2), 135. https://doi.org/10.3390/agriculture11020135