1. Introduction
Environmental pollution is one of the most serious problems that affects society in the 21st century. Among these difficulties, water contamination by heavy metals is one of the current problems that originates from several human activities, such as wearing to geological process; mining; and industries such as microelectronics, electroplating, battery manufacturing, dyes, chemical products and pharmaceutical products [
1,
2,
3]. The heavy metals are one of the main pollutants of water, being highly toxic for human beings. It is well-known that the most abundant heavy metals in water are mercury (Hg) [
4], nickel (Ni) [
5], copper (Cu) [
6], plumb (Pb) [
7] and chromium (Cr) [
8]. Various technologies have been used for the removal of metal ions, among which the following stand out: chemical precipitation, chemical coagulation, oxidation, reduction, ion exchange, filtration, ultrafiltration, nanofiltration, adsorption (activated carbon, zeolites, silica gel), technologies membrane (reverse osmosis), electrochemical treatment (electrodialysis and electrocoagulation) and application of artificial wetlands (some stabilization), among others, resulting in many cases ineffective and expensive [
9,
10]. Consequently, it is important to develop new technologies for the detoxification of said effluents and chemical stabilization of the compound [
11]. This is the reason for the development of new technologies based on national raw materials is a pending task for researchers from developing countries like Colombia.
In Colombia, because of its different climates, there is a great variety of possible materials with potential applications as lignocellulosic material. Among the most used materials is banana skin, which has been employed to remove metals such as Cu (II), Zn (II), Co (II), Ni (II) and Pb (II) [
12] with 49.14% efficiency; other natural material used is the coffee rusk, that is employed in process for removing Cu (II), Zn (II) and Cd (II) [
13] with 58.45% efficiency. Additionally, using sheep’s wool, uranium has been removed [
14] and with the rice husk, metals like Cd (II), Zn (II), Co (II), Ni (II), Mn (II) and Hg (II) [
15] can be removed with 62.85% efficiency; additionally, using the rice husk, there are heavy metal removal reports such as Cd (II), Cu (II), Cr (VI), Pb (II), Zn (II) and As (III) with adsorption capacities between 72.80% and 99.30% [
16]. In most previous reports, an additional process of the material is required. Generally, a chemical process is conducted to activate the material, leaving impurities that generate a subsequent contamination [
17].
Modeling and simulation processes are a very useful tool because, regard independently of the complexity of the bio filter system, it can be modeled considering most of the variables for reaching an efficient operation. Within the theoretical studies, it is found that the mathematical modeling of a heavy metal adsorption system can evaluate the dynamics regarding the ways that metals adhere to the lignocellulosic material [
18]. Additionally, it was found that with a vertical flow filter, a dynamic simulation can be performed to evaluate the transport and destination of heavy metals in wastewater. For modeling systems that remove heavy metals, the following characteristics are considered: volume of residual water, temperature, concentration of heavy metals, contact time and flow [
19]. Moreover, it is required to consider an initial concentration, contact time [
20], porosity, depth, filtration rate, loss of load, geometry, inlet and outlet pressure and the characteristics of the affluent [
21]. Furthermore, the pH is considered as a fundamental variable, because at high values, the negative sites increase and at low values, the formation of hydroxylated ions decreases as well as the adsorption of heavy metals [
22]. Employing a statistical model is proposed for the analysis of adsorption; they found that for the filtering process, a controlled temperature must be maintained, avoiding the deterioration of the system used as a filter [
23]; and the efficiency of a filter depends on the characteristics and properties of interactions between the filter and the substance or element that will be retained.
Contaminants are not only found in water; for example, Kalawik et al., [
24] reported that heavy metals such as Hg, Cd, Pb, As and Ni have been found in foods such as sushi, the concentrations of heavy metals found are high enough to affect the health of people who eat these foods. Traces of Pb, Cu, Cd and Hg have also been found in meat sold for human consumption [
25]. Other studies show how heavy metals such as Cd, Pb and as have been the most detected in foods such as vegetables and fruits, presenting a great risk to humans. Due to the large presence of heavy metals both in water and in food, the need arises to propose alternatives for the removal of heavy metals. The alternative that arises in this case is a bio-filter made from easily accessible materials that contribute to a circular economy, such as rice husk, which allows the removal of metals such as Cr (VI), Cd (II) and Cu (II) from water. In this work, kinetic models were used for modelling the effectiveness of rice husk as a material used in a filter for the removal of Cr (VI), Cd (II) and Cu (II) in batch processes. The outline of this document is the following: In
Section 2, we give the model, the bio filter design, and the explication of selection of lignocellulosic material. In
Section 3, isotherms and breaking curves for each heavy metal are presented. Finally, conclusions are given in
Section 4.
4. Discussion
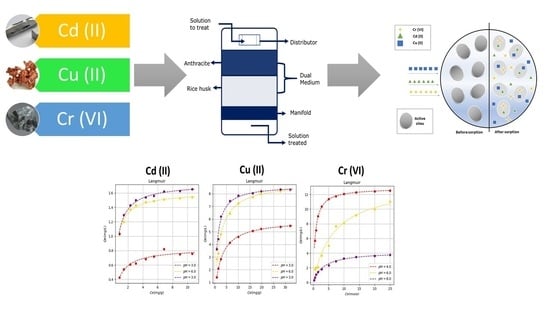
Varying the temperature, taking the Langmuir isotherm as a reference, it indicates that the adsorption process occurs when a monolayer of the absorbed metal is formed, reaching a saturation point until a quantity of the metal is absorbed. In addition, it indicates that there is an interaction between the molecules adsorbed on the surface of the rice husk, the interactions happen by mechanism such as ion exchange. It is also observed that adsorption occurs at specific active sites that are located on the surface of the rice husk as shown in the filter’s operating scheme (
Figure 2). Regarding the coefficients that describe the behavior of the Langmuir isotherm, we have b that represents the affinity of the binding sites, which for each metal presents its maximum value at room temperature (298.15 K) and
is the maximum capacity of adsorption of the bio sorbent, which increases with the temperature value showing that up to 303.15 K the bio-filter works correctly, This is due to the fact that at the reported temperatures (293.15, 298.15 and 303.15 K) the lignocellulosic material (rice husk) does not deteriorate and does not lose its absorption capacity, if the temperature were increased, there could be inconveniences in terms of to the functioning of the bio-filter that could be evidenced in its adsorption capacity.
According to the isotherms varying the pH, these isotherms describe adsorption processes with microporous solids, which have a very fine porous structure as rice husk. These results indicate that there is a formation of a layer composed of the heavy metal removed, which in this case can be Cd (II), Cu (II) or Cr (VI), which occurs in adsorption. The pH values that favor removal are higher than 4.5; for Cd (II) and Cu (II), this behavior is observed. On the other hand, for Cr (VI) absorption is favored at pH values lower than 4, this is since Cr (VI) has different valences and in some cases, it behaves like Cr (III).
According to the rupture curves it is obtained that the capacity at the breaking point increases with the increase in height. The cycle time exhibits typical values between 2 and 24 h; after this time, the lignocellulosic material stops filtering with the same efficiency. These results indicate that useful height values may be 0.55 m for Cu (II) and Cd (II). If the height values are reduced, the effect of the axial dispersion phenomenon in the mass transfer predominates; therefore, the metal ions do not have enough time to diffuse throughout the mass of the adsorbent. With a major height, the surface area of the adsorbent contains more binding sites for adsorption. In this type of filter, the water flows through a bed of gravel and sand for long distances, which increases the probability of the solid has contact with other suspended particles, and with the medium formed on the surface of the gravel granule or sand, being thus retained between the filtering material. Later, heavy metals are suspended in the lignocellulosic material. The metal ions must have enough time to be removed and thus im-prove the adsorption capacity of the lignocellulosic material which in this case is the rice husk. Therefore, the higher height favors the removal of heavy metals. For the study of the Cu (II) removal in a theoretical way, different bed heights were evaluated, finding that the bed height can only determine the total sorption capacity of a fixed bed, which is directly related to the length of service time. This implies that the bed height is one of the main operational factors that control the effluent concentrations of target contaminants in practical field applications [
56].
For Cr (VI), the removal is less efficient, since a smaller height should be used compared with Cd (II) and Cu (II). The chromium may be retained in the following forms: adsorbed (Cr (VI) and Cr (III)), precipitated and bioaccumulated in the support, in the cell membrane and in the metabolites produced by microorganisms [
57]. Therefore, in the case of Cr (VI) it is not enough only to make changes in the height, they must be considered in the other forms that Cr (VI) can be presented since it decomposes in several ways.
5. Conclusions
The application of bio-adsorption in the purification of waters polluted by heavy metals presents a great potential, since lignocellulosic materials can be obtained in large quantities, are cheap and can selectively remove Cd (II), Cu (II) and Cr (VI) of aqueous solutions. The results obtained allow to conclude that rice husk is a good alternative for the implementation of filters with the capacity to remove Cd (II), Cu (II) and Cr (VI) with efficiencies of 83.21%, 67.11% and 92.18%, respectively, for certain values of the height of the filter, temperature and pH. At higher height values, the rupture curves show that the removal process occurs over a longer period and therefore the process is more efficient. Except for Cr (VI) which, depending on the interactions with the medium, can be converted into Cr (III) and presents a different behavior than for Cd (II) and Cu (II).
Perspectives
The simulation of the bio-filter using the Thomas model, Bohart-Adams model and Yoon-Nelson model is anticipated as future work. The above, in order to compare the operating parameters of the filter A, possible real-world applications would be in the tributaries near the mining areas in Colombia in regions such as Cordoba, Antioquia or Norte de Santander, which have, as a product or heavy metal by-product, Cd (II), Cu (II) and Cr (VI). It is proposed to use in these regions because they are the ones with the highest concentration of heavy metals in the country.