Nutrient Composition, Forage Parameters, and Antioxidant Capacity of Alfalfa (Medicago sativa, L.) in Response to Saline Irrigation Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material and Growth Conditions
2.2. Plant Growth and Nutrient Composition
| Treatment | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| EC (dS·m−1) | 3.1 | 7.2 | 12.7 | 18.4 | 24.0 |
| Ion Concentration in mmolc·L−1 | |||||
| Ca2+ | 6.4 | 19.2 | 25.0 | 29.4 | 28.4 |
| Mg2+ | 4.0 | 14.3 | 24.1 | 40.7 | 58.5 |
| Na+ | 15.5 | 54.2 | 101 | 169 | 229 |
| K+ | 6.4 | 6.4 | 6.2 | 6.4 | 6.6 |
| SO42− | 15.3 | 53.3 | 85.0 | 132 | 182 |
| Cl− | 8.0 | 31.8 | 62.9 | 104 | 133 |
| PO43− | 0.3 | 0.3 | 0.3 | 0.4 | 0.5 |
| NO3− | 5.5 | 5.6 | 5.5 | 6.0 | 6.0 |
2.3. Oxygen Radical Absorbance Capacity (ORAC) and Total Phenolics (TP) Analyses
2.4. Forage Quality
2.5. Statistical Analysis
3. Results
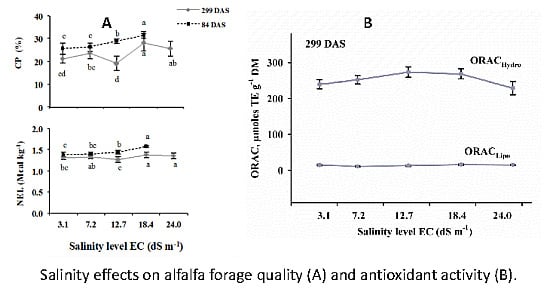
3.1. Forage Quality

3.2. Nutrient Composition of Alfalfa
3.2.1. Macronutrients
| N | P | K | Ca | Mg | Total S | |
|---|---|---|---|---|---|---|
| DM (g·kg−1) | ||||||
| EC dS·m−1 Second Harvest (84 DAS) | ||||||
| 3.1 | 40.8 c ± 1.43 | 2.6 b ± 0.09 | 46.4 a ± 1.05 | 14.1 a ± 0.4 | 2.6 c ± 0.14 | 3.5 d ± 0.08 |
| 7.2 | 42.1 c ± 1.04 | 2.7 b ± 0.09 | 41.4 b ± 0.94 | 13.5 a ± 0.5 | 2.7 c ± 0.16 | 3.9 c ± 0.10 |
| 12.7 | 46.0 b ± 0.56 | 2.9 b ± 0.08 | 38.6 c ± 0.62 | 13.0 c ± 0.69 | 3.4 b ± 0.22 | 4.8 b ± 0.20 |
| 18.4 | 50.5 a ± 0.80 | 3.8 a ± 0.13 | 34.3 d ± 0.88 | 12.1 b ± 0.24 | 4.8 a ± 0.07 | 7.4 a ± 0.17 |
| 24 | ND | ND | ND | ND | ND | ND |
| Seventh Harvest (299 DAS) | ||||||
| 3.1 | 34.1 d ± 1.07 | 3.4 b ± 0.17 | 40.3 a ± 1.12 | 18.0 a ± 0.51 | 2.5 c ± 0.08 | 3.8 a ± 0.12 |
| 7.2 | 37.6 bc ±1.37 | 3.1 b ± 0.06 | 30.4 bc ± 0.74 | 18.3 a ± 0.61 | 2.8 bc ± 0.12 | 4.6 a ± 0.20 |
| 12.7 | 30.8 d ± 1.77 | 2.8 b ± 0.14 | 31.0 b ± 0.68 | 16.7 a ± 0.51 | 3.2 ab ± 0.12 | 4.8 a ± 0.15 |
| 18.4 | 45.3 a ± 2.11 | 4.1 a ± 0.12 | 27.3 cd ± 0.56 | 12.1 b ± 0.45 | 3.0 bc ± 0.10 | 4.8 a ± 0.15 |
| 24 | 40.8 a ±1.92 | 4.3 a ±0.16 | 26.7 d ± 0.61 | 11.0 b ± 0.83 | 3.6 a ± 0.20 | 5.3 a ± 0.39 |
3.2.2. Micronutrients
| Fe | Cu | Mn | Zn | Mo | |
|---|---|---|---|---|---|
| DM (mg·kg−1) | |||||
| EC dS·m−1 Second Harvest (84 DAS) | |||||
| 3.1 | 104.0 a ± 6.29 | 2.1 a ± 0.27 | 25.5 b ± 3.38 | 40.9 d ± 1.32 | 2.0 c ± 0.09 |
| 7.2 | 99.1 a ± 4.90 | 2.3 a ± 0.10 | 31.7 ab ± 4.8 | 45.9 c ± 1.00 | 3.1 b ± 0.11 |
| 12.7 | 106.5 a ± 5.89 | 3.1 a ± 0.16 | 34.8 a ± 4.10 | 54.9 b ± 1.11 | 3.2 b ± 0.14 |
| 18.4 | 109.6 a ± 5.0 | 3.1 a ± 0.19 | 34.8 a ± 1.10 | 60.5 a ± 1.25 | 4.1 a ± 0.11 |
| 24 | ND | ND | ND | ND | ND |
| Seventh Harvest (299 DAS) | |||||
| 3.1 | 116.1 a ± 6.35 | 5.8 a ±0.83 | 17.2 b ± 0.91 | 97.6 a ± 3.36 | 2.7c ± 0.19 |
| 7.2 | 97.7 b ± 7.35 | 6.1 a ± 0.64 | 24.6 a ± 1.44 | 89.9 a ± 3.26 | 6.4 a ± 0.43 |
| 12.7 | 89.9 b ± 7.35 | 6.5 a ± 0.41 | 18.9 b ± 0.99 | 105.6 a ± 3.18 | 6.3 a ± 0.44 |
| 18.4 | 83.5 b ± 3.17 | 5.3 a ± 0.26 | 17.4 b ± 1.05 | 101.3 a ± 3.26 | 4.7 c ± 0.36 |
| 24 | 92.3 b ± 7.69 | 5.7 a ± 0.49 | 14.8 b ± 1.04 | 98.3 a ± 3.85 | 4.2 c ± 0.21 |
3.3. Antioxidant Capacity of Alfalfa
| EC | ORACLipo | ORACHydro | ORACTotal |
|---|---|---|---|
| (dS·m−1) | (μmoles·TE·g−1 DM) | ||
| 3.1 | 15.0 ± 2.4 | 239.5 ± 12.8 | 254.5 ± 13.6 |
| 7.2 | 11.2 ± 1.5 | 252.1 ± 11.6 | 263.3 ± 11.0 |
| 12.7 | 13.4 ± 2.5 | 273.6 ± 14.3 | 286.9 ± 14.2 |
| 18.4 | 16.4 ± 1.7 | 268.4 ± 14.0 | 284.8 ± 15.5 |
| 24.0 | 15.3 ± 1.0 | 228.8 ± 18.3 | 244.1 ± 18.2 |

4. Discussion
4.1. Forage Quality
4.2. Mineral Nutrient Composition
4.3. Antioxidant Capacity of Alfalfa
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADF | acid detergent fiber |
| NDF | neutral detergent fiber |
| NEL | net energy for lactation |
| CP | crude protein |
| RFV | relative feed value |
| ORAC | oxygen radical absorbance capacity |
| TP | total phenolics |
References
- Radović, J.; Sokolović, D.; Marković, J. Alfalfa—Most important perenial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- USDA-ARS. Roadmap for Alfalfa Research. Available online: http://ars.usda.gov/SP2UserFiles/Place/54281000/alfalfaroadmap2.pdf (accessed on 11 March 2014).
- DePeters, E. Forage Quality: Important Attributes & Changes on the Horizon. In the Proceedings of California Alfalfa and Grains Symposium, Sacramento, CA, USA, 10–12 December 2012; UC Cooperative Extension, Plant Sciences Department, University of California, Davis: Davis, CA, USA, 2012. [Google Scholar]
- Khorasani, G.R.; Janzen, R.A.; McGill, W.B.; Kennelly, J.J. Site and extent of mineral absorption in lactating cows fed whole-crop cereal grain silage of alfalfa silage. J. Anim.Sci. 1997, 75, 239–248. [Google Scholar] [PubMed]
- Smedema, L.K.; Shiati, K. Irrigation and salinity: A perspective review of the salinity hazards of irrigation development in the arid zone. Irrig. Drain. Syst. 2002, 16, 161–174. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Al-Khatib, M.; McNeilly, T.; Collins, J.C. The potential of selection and breeding for improved salt tolerance in lucerne (Medicago sativa L.). Euphytica 1992, 65, 43–51. [Google Scholar] [CrossRef]
- Mass, E.V.; Grattan, S.R. Crop yields as affected by salinity. In Agricultural Drainage; Agron. Monograph 38; Skaggs, R.W., van Schilfgaarde, J., Eds.; ASA, CSSA, SSA: Madison, WI, USA, 1999; pp. 55–108. [Google Scholar]
- Robinson, P.H.; Grattan, S.R.; Getachew, G.; Grieve, C.M.; Poss, J.A.; Suarez, D.L.; Benes, S.E. Biomass accumulation and potential nutritive value of some forages irrigated with saline-sodic drainage water. Anim. Feed Sci. Technol. 2004, 111, 175–189. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M.; Poss, J.A.; Robinson, P.H.; Suarez, D.L.; Benes, S.E. Evaluation of salt-tolerant forages for sequential water reuse systems: I. Biomass production. Agric. Water Manag. 2004, 70, 109–120. [Google Scholar] [CrossRef]
- Suyama, H.; Benes, S.E.; Robinson, P.H.; Grattan, S.R.; Grieve, C.M.; Getachew, G. Forage yield and quality under irrigation with saline-sodic drainage water: Greenhouse evaluation. Agric. Water Manage. 2007, 88, 159–172. [Google Scholar] [CrossRef]
- Steppuhn, H.; Acharya, S.N.; Iwaasa, A.D.; Gruber, M.; Miller, D.R. Inherent responses to root-zone salinity in nine alfalfa populations. Can. J. Plant Sci. 2012, 92, 235–248. [Google Scholar] [CrossRef]
- Rubio, M.C.; Bustos-Sanmamed, P.; Clemente, M.R.; Becana, M. Effects of salt stress on the expression of antioxidant genes and proteins in the model legume Lotus japonicus. New Phytol. 2009, 181, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, H.; Fotopoulos, V.; Mylona, P.V.; Jebara, M.; Elarbi Aouani, M.; Polidoros, A.N. Antioxidant gene–enzyme responses in Medicago truncatula genotypes with different degree of sensitivity to salinity. Physiol. Plant. 2011, 141, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Stochmal, A.; Oleszek, W. Seasonal and structural changes in flavones in alfalfa (Medicago sativa) aerial parts. Int. J. Food Agric. Environ. 2007, 5, 170–174. [Google Scholar]
- Choi, K.C.; Hwang, J.M.; Bang, S.J.; Kim, B.T.; Kim, D.H.; Chae, M.; Lee, S.A.; Choi, G.J.; Kim, D.H.; Lee, J.C. Chloroform extract of alfalfa (Medicago sativa) inhibits lipopolysaccharide-induced inflammation by downregulating ERK/NF-κB signaling and cytokine production. J. Medic. Food 2013, 16, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm. Biol. 2011, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Cornacchione, M.V.; Suarez, D.L. Emergence, forage production, and ion relations of alfalfa in response to saline waters. Crop Sci. 2015, 55, 444–457. [Google Scholar] [CrossRef]
- Suarez, D.L.; Simunek, J. Unsatchem: Unsaturated water and solute transport model with equilibrium and kinetic chemistry. Soil Sci. Soc. Am. J. 1997, 61, 1633–1646. [Google Scholar] [CrossRef]
- Kalu, B.A.; Fick, G. Quantifying morphological development of alfalfa for studies of herbage quality. Crop Sci. 1981, 21, 267–271. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity [oxygen radical absorbance capacity (ORAC)] of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; p. 2000. [Google Scholar]
- National Forage Testing Association. Forage Analysis Procedures. Available online: http://www.foragetesting.org/files/LaboratoryProcedures.pdf (accessed on 27 May 2013).
- Atwater, W.O.; Bryant, A.P. The Chemical Composition of American Food Materials; USDA Office of Experiment Stations, Ed.; US Government Printing Office: Washington, DC, USA, 1906; p. 87.
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. Infostat. Grupo Infostat; FCA Universidad Nacional de Córdoba, Argentina. Available online: Http://www.Infostat.Com.Ar (accessed on 30 August 2013).
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Getachew, G.; Pittroff, W.; DePeters, E.J.; Putnam, D.H.; Dandekar, A.; Goyal, S. Influence of tannic acid application on alfalfa hay: In vitro rumen fermentation, serum metabolites and nitrogen balance in sheep. Animal 2008, 2, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Minson, D.J. Forage in Ruminant Nutrition; Academic Press: San Diego, CA, USA, 1990; p. 463. [Google Scholar]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Putnam, D.H.; Robinson, P.; DePeters, E. Forage quality and testing. In Irrigated Alfalfa Management for Mediterranean and Desert Zones; Publication 3512; Summers, C.G., Putnam, D.H., Eds.; University of California/Agricultural and Natural Resources: Davis, CA, USA, 2008; pp. 241–264. [Google Scholar]
- Lemaire, G.; Avice, J.C.; Kim, T.H.; Ourry, A. Developmental changes in shoot N dynamics of lucerne (Medicago sativa L.) in relation to leaf growth dynamics as a function of plant density and hierarchical position within the canopy. J. Exp. Bot. 2005, 56, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, G.; Khaity, M.; Onillon, B.; Allirand, J.M.; Chartier, M.; Gosse, G. Dynamics of accumulation and partitioning of N in leaves, stems and roots of lucerne (Medicago sativa L.) in a dense canopy. Ann. Bot. 1992, 70, 429–435. [Google Scholar]
- Hoffman, G.J.; Maas, E.V.; Rawlins, S.L. Salinity-ozone interactive effects on alfalfa yield and water relations. J. Environ. Qual. 1975, 4, 326–331. [Google Scholar] [CrossRef]
- Mueller, S.C.; Teuber, L.R. Alfalfa growth and development. In Irrigated Alfalfa Management for Mediterranean and Desert Zones; Publication 3512; Summers, C.G., Putnam, D.H., Eds.; University of California/Agricultural and Natural Resources: Davis, CA, USA, 2008; pp. 31–38. [Google Scholar]
- Orloff, S.B.; Putnam, D.H. Harvest strategies for alfalfa. In Irrigated Alfalfa Management for Mediterranean and Desert Zones; Publication 3512; Summers, C.G., Putnam, D.H., Eds.; University of California/Agricultural and Natural Resources: Davis, CA, USA, 2008; pp. 197–207. [Google Scholar]
- Yurtseven, S. The nutrient and energy contents of medicago varieties growth in salt-affected soils of the harran plain. Hayvansal Üretim 2011, 52, 39–45. [Google Scholar]
- USDA-CO, D.O.A.M.N.S. California hay report. Available online: http://www.ams.usda.gov/mnreports/ml_gr311.txt (accessed on 9 May 2013).
- Hussain, G.; Al-Jaloud, A.A.; Ai-Shammary, S.F.; Karimulla, S. Effect of saline irrigation on the biomass yield, and the protein, nitrogen, phosphorus, and potassium composition of alfalfa in a pot experiment. J. Plant Nutr. 1995, 18, 2389–2408. [Google Scholar] [CrossRef]
- Isla, R.; Aragüés, R. Response of alfalfa (Medicago sativa L.) to diurnal and nocturnal saline sprinkler irrigations. I: Total dry matter and hay quality. Irrig. Sci. 2009, 27, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Halim, R.A.; Buxton, D.R.; Hattendorf, M.J.; Carlson, R.E. Water-stress effects on alfalfa forage quality after adjustment for maturity differences. Agron. J. 1989, 81, 189–194. [Google Scholar] [CrossRef]
- Pessarakli, M.; Huber, J.T. Biomass production and protein synthesis by alfalfa under salt stress. J. Plant Nutr. 1991, 14, 283–293. [Google Scholar] [CrossRef]
- Meyer, R.D.; Marcum, D.B.; Orloff, S.B.; Schmierer, J.L. Alfalfa fertilization strategies. In Irrigated Alfalfa Management for Mediterranean and Desert Zones; Publication 3512; Summers, C.G., Putnam, D.H., Eds.; University of California/Agricultural and Natural Resources: Davis, CA, USA, 2008; pp. 73–87. [Google Scholar]
- te Velde, G. Milking Jersey’s vs. Holstein’s on a Commercial Dairy in California: Milk Production, Feed Efficiency, Intake, Costs, and Advantages; BS, California Politechnic State University: San Luis Obispo, CA, USA, 2013. [Google Scholar]
- Koong, L.-J.; Wise, M.B.; Barrick, E.R. Effect of elevated dietary levels of iron on the performance and blood constituents of calves. J. Anim. Sci. 1970, 31, 422–427. [Google Scholar] [PubMed]
- Ammerman, C.B.; Goodrich, R.D. Advances in mineral nutrition in ruminants. J. Anim. Sci. 1983, 57, 519–533. [Google Scholar] [PubMed]
- Fisher, L.J.; Dinn, N.; Tait, R.M.; Shelford, J.A. Effect of level of dietary potassium on the absorption and excretion of calcium and magnesium by lactating cows. Can. J. Anim. Sci. 1994, 74, 503–509. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M.; Poss, J.A.; Robinson, P.H.; Suarez, D.L.; Benes, S.E. Evaluation of salt-tolerant forages for sequential water reuse systems: III. Potential implications for ruminant mineral nutrition. Agric. Water Manage. 2004, 70, 137–150. [Google Scholar] [CrossRef]
- Grunes, D.L.; Stout, P.R.; Brownell, J.R. Grass tetany of ruminants. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: London, UK, 1970; Volume 22, pp. 331–374. [Google Scholar]
- Jittakhot, S.; Schonewille, J.T.; Wouterse, H.; Focker, E.J.; Yuangklang, C.; Beynen, A.C. Effect of high magnesium intake on apparent magnesium absorption in lactating cows. Anim. Feed Sci. Technol. 2004, 113, 53–60. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.A.; Grattan, S.R.; Suarez, D.L.; Benes, S.E.; Robinson, P.H. Evaluation of salt-tolerant forages for sequential water reuse systems: II. Plant–ion relations. Agric. Water Manage. 2004, 70, 121–135. [Google Scholar] [CrossRef]
- Arthington, J. Know the Sulfur Content of Your Forage—Test It. Available online: http://rcrec-ona.ifas.ufl.edu/pdf/publications/ona-reports/2013/5%202013/or5-13.html (accessed on 9 May 2013).
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Corah, L. Trace mineral requirements of grazing cattle. Anim. Feed Sci. Technol. 1996, 59, 61–70. [Google Scholar] [CrossRef]
- Scaletti, R.W.; Amaral-Phillips, D.M.; Harmon, R.J. Using Nutrition to Improve Immunity Against Disease in Dairy Cattle: Copper, Zinc, Selenium, and Vitamin E; University of Kentucky: Lexington, KY, USA, 1999; pp. 1–4. [Google Scholar]
- Jones, M.; van der Merwe, D. Copper Toxicity in Sheep is on the Rise in Kansas and Nebraska; Kansas State University/Veterinary Medical Teaching Hospital: Manhattan, KS, USA, 2008; p. 5. [Google Scholar]
- Stochmal, A.; Piacente, S.; Pizza, C.; De Riccardis, F.; Leitz, R.; Oleszek, W. Alfalfa (Medicago sativa L.) flavonoids. 1. Apigenin and luteolin glycosides from aerial parts. J. Agric. Food Chem. 2001, 49, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Experim. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Ferreira, J.F.S. Artemisia Species in Small Ruminant Production: Their Potential Antioxidant and Anthelmintic Effects. In Appalachian Workshop and Research Update: Improving Small Ruminant Grazing Practices; Morales, M., Ed.; Mountain State University/USDA: Beaver, WV, USA, 2009; pp. 53–70. [Google Scholar]
- Katiki, L.M.; Ferreira, J.F.S.; Gonzalez, J.M.; Zajac, A.M.; Lindsay, D.S.; Chagas, A.C.S.; Amarante, A.F.T. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet. Parasitol. 2013, 192, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S. In vitro and in vivo antioxidant activity of alfalfa (Medicago sativa L.) on carbon tetrachloride intoxicated rats. Am. J. Chin. Med. 2012, 40, 779. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Luthria, D.L. Drying affects artemisinin, dihydroartemisinic acid, artemisinic acid, and the antioxidant capacity of Artemisia annua L. Leaves. J. Agric. Food Chem. 2010, 58, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Tekippe, J.A.; Hristov, A.N.; Heyler, K.S.; Cassidy, T.W.; Zheljazkov, V.D.; Ferreira, J.F.S.; Karnati, S.K.; Varga, G.A. Rumen fermentation and production effects of Origanum vulgare L. Leaves in lactating dairy cows. J. Dairy Sci. 2011, 94, 5065–5079. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.F.S.; Cornacchione, M.V.; Liu, X.; Suarez, D.L. Nutrient Composition, Forage Parameters, and Antioxidant Capacity of Alfalfa (Medicago sativa, L.) in Response to Saline Irrigation Water. Agriculture 2015, 5, 577-597. https://doi.org/10.3390/agriculture5030577
Ferreira JFS, Cornacchione MV, Liu X, Suarez DL. Nutrient Composition, Forage Parameters, and Antioxidant Capacity of Alfalfa (Medicago sativa, L.) in Response to Saline Irrigation Water. Agriculture. 2015; 5(3):577-597. https://doi.org/10.3390/agriculture5030577
Chicago/Turabian StyleFerreira, Jorge F. S., Monica V. Cornacchione, Xuan Liu, and Donald L. Suarez. 2015. "Nutrient Composition, Forage Parameters, and Antioxidant Capacity of Alfalfa (Medicago sativa, L.) in Response to Saline Irrigation Water" Agriculture 5, no. 3: 577-597. https://doi.org/10.3390/agriculture5030577
APA StyleFerreira, J. F. S., Cornacchione, M. V., Liu, X., & Suarez, D. L. (2015). Nutrient Composition, Forage Parameters, and Antioxidant Capacity of Alfalfa (Medicago sativa, L.) in Response to Saline Irrigation Water. Agriculture, 5(3), 577-597. https://doi.org/10.3390/agriculture5030577