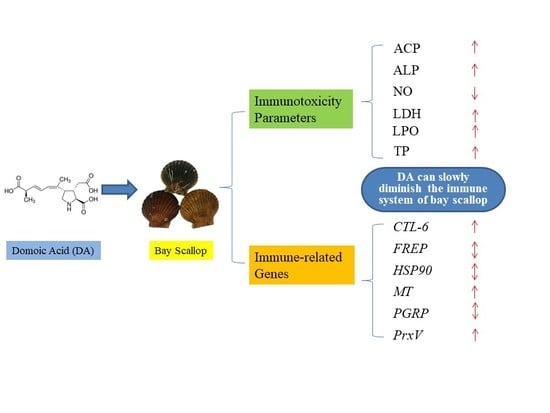
Effects of Marine Toxin Domoic Acid on Innate Immune Responses in Bay Scallop Argopecten irradians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Domoic Acid
2.2. Animals
2.3. Measurement of Non-Specific Immune Responses
2.4. Real-Time Quantitative PCR Analyses of Gene Expression
2.5. Statistical Analyses
3. Results
3.1. Non-Specific Immune Responses
3.1.1. ACP Activity
3.1.2. ALP Activity
3.1.3. LDH Activity
3.1.4. LPO Level
3.1.5. NO Level
3.1.6. Total Protein Concentration
3.2. Expression of Immune-Related Genes
3.2.1. Expression of CTL6
3.2.2. Expression of FREP
3.2.3. Expression of HSP90
3.2.4. Expression of MT
3.2.5. Expression of PGRP
3.2.6. Expression of PrxV
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mazmancı, B.; Çavas, T. Antioxidant enzyme activity and lipid peroxidation in liver and gill tissues of Nile tilapia (Oreochromis niloticus) following in vivo exposure to domoic acid. Toxicon 2010, 55, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.M.; Lopes, A.R.; Costa, P.; Rosa, R. Cephalopods as vectors of harmful algal bloom toxins in marine food webs. Mar. Drugs 2013, 11, 3381–3409. [Google Scholar] [CrossRef] [PubMed]
- Ventoso, P.; Pazos, A.J.; Pérez-Parallé, M.L.; Blanco, J.; Triviño, J.C.; Sánchez, J.L. RNA-Seq transcriptome profiling of the queen scallop (Aequipecten opercularis) digestive gland after exposure to domoic acid-producing Pseudo-nitzschia. Toxins 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Tilton, S.C.; Bammler, T.K.; Beyer, R.P.; Srinouanprachan, S.; Stapleton, P.L.; Farin, F.M.; Gallagher, E.P. Gene expression profiles in zebrafish brain after acute exposure to domoic acid at symptomatic and asymptomatic doses. Toxicol. Sci. 2009, 107, 65–77. [Google Scholar] [CrossRef]
- Nogueira, I.; Lobo-da-Cunha, A.; Afonso, A.; Rivera, S.; Azevedo, J.; Monteiro, R.; Cervantes, R.; Gago-Martinez, A.; Vasconcelos, V. Toxic effects of domoic acid in the seabream Sparus aurata. Mar. Drugs 2010, 8, 2721–2732. [Google Scholar] [CrossRef]
- Zabaglo, K.; Chrapusta, E.; Bober, B.; Kaminski, A.; Adamski, M.; Bialczyk, J. Environmental roles and biological activity of domoic acid: A review. Algal Res. 2016, 13, 94–101. [Google Scholar] [CrossRef]
- Pazos, A.J.; Ventoso, P.; Martínez-Escauriaza, R.; Pérez-Parallé, M.L.; Blanco, J.; Triviño, J.C.; Sánchez, J.L. Transcriptional response after exposure to domoic acid-producing Pseudo-nitzschia in the digestive gland of the mussel Mytilus galloprovincialis. Toxicon 2017, 140, 60–71. [Google Scholar] [CrossRef]
- Song, L.S.; Wang, L.L.; Zhang, H.; Wang, M.Q. The immune system and its modulation mechanism in scallop. Fish. Shellfish Immunol. 2015, 46, 65–78. [Google Scholar] [CrossRef]
- Suresh, K.; Mohandas, A. Hemolymph acid phosphatase activity pattern in copper-stressed bivalves. J. Invertebr. Pathol. 1990, 55, 118–125. [Google Scholar] [CrossRef]
- Chi, C.; Yun, S.; Giri, S.S.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Park, S.C. Effect of the algicide thiazolidinedione 49 on immune responses of bay scallop Argopecten Irradians. Molecules 2019, 24, 3579. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C. The Role of Lysosomal Hydrolases in Molluscan Cellular Response to Immunologic Challenge, 1st ed.; Springer: Boston, MA, USA, 1978; pp. 59–71. [Google Scholar]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Yun, S.; Kim, S.G.; Park, S.C. Marine toxin okadaic acid affects the immune function of bay scallop (Argopecten irradians). Molecules 2016, 21, 1108. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Wan, P.; Bernstein, L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control 2001, 12, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhou, Z.; Wang, L.L.; Shi, X.W.; Wang, J.J.; Yue, F.; Yi, Q.L.; Yang, C.Y.; Song, L.S. The immunomodulation of inducible nitric oxide in scallop Chlamys farreri. Fish. Shellfish Immunol. 2013, 34, 100–108. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, Z.; Wang, L.L.; Wang, L.L.; Yue, F.; Wang, L.L.; Song, L.S. A scallop nitric oxide synthase (NOS) with structure similar to neuronal NOS and its involvement in the immune defense. PLoS ONE 2013, 8, e69158. [Google Scholar] [CrossRef]
- Franchini, A.; Fontanili, P.; Ottaviani, E. Invertebrate immunocytes: Relationship between phagocytosis and nitric oxide production. Comp. Biochem. Physiol. B 1995, 110, 403–407. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, J.M.; Song, L.S.; Qiu, L.M.; Yu, Y.D.; Zhang, H.; Ni, D.J. Molecular cloning, characterization and expression of heat shock protein 90 gene in the haemocytes of bay scallop Argopecten irradians. Fish. Shellfish Immunol. 2008, 24, 379–385. [Google Scholar] [CrossRef]
- Liu, H.; Kelly, M.S.; Campbell, D.A.; Fang, J.G.; Zhu, J.X. Accumulation of domoic acid and its effect on juvenile king scallop Pecten maximus (Linnaeus, 1758). Aquaculture 2008, 284, 224–230. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Todd, E.C.D. Domoic acid and amnesic shellfish poisoning—A review. J. Food Prot. 1993, 56, 69–83. [Google Scholar] [CrossRef]
- Wells, M.L.; Trick, C.G.; Cochlan, W.P.; Hughes, M.P.; Trainer, V.L. Domoic acid: The synergy of iron, copper, and the toxicity of diatoms. Limnol. Oceanogr. 2005, 50, 1908–1917. [Google Scholar] [CrossRef] [Green Version]
- Douglas, D.J.; Kenchington, E.R.; Bird, C.J.; Pocklington, R.; Bradford, B.; Silvert, W. Accumulation of domoic acid by the sea scallop (Placopecten magellanicus) fed cultured cells of toxic Pseudo-nitzschia multiseries. Fish. Aquat. Sci. 1997, 54, 907–913. [Google Scholar] [CrossRef]
- Jing, G.; Li, Y.; Xie, L.; Zhang, R. Metal accumulation and enzyme activities in gills and digestive gland of pearl oyster (Pinctada fucata) exposed to copper. Comp. Biochem. Physiol. C 2006, 144, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Kim, S.W.; Yun, S.; Park, S.C. Effects of algal toxin okadaic acid on the non-specific immune and antioxidant response of bay scallop (Argopecten irradians). Fish. Shellfish Immunol. 2017, 65, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gestal, C.; Roch, P.; Renault, T.; Pallavicini, A.; Paillard, C.; Novoa, B.; Oubella, R.; Venier, P. Study of diseases and the immune system of bivalves using molecular biology and genomics. Rev. Fish. Sci. 2008, 16, 131–154. [Google Scholar] [CrossRef]
- Barbosa, D.B.; Mello, A.D.A.; Allodi, S.; Barros, C.M.D. Acute exposure to water-soluble fractions of marine diesel oil: Evaluation of apoptosis and oxidative stress in an ascidian. Chemosphere 2018, 211, 308–315. [Google Scholar] [CrossRef]
- Pande, M.; Harps, A.; Sundaram, M.; Vig, P.J.S. Role of nitric oxide in domoic acid induced hippocampal degeneration. J. Neurol. Sci. Turk. 2007, 24, 16–24. [Google Scholar]
- Senthil-Nathan, S.; Kalaivani, K.; Chung, P.G.; Murugan, K. Effect of neem limonoids on lactate dehydrogenase (LDH) of the rice leaffolder, Cnaphalocrocis medinalis (Guenée) (Insecta: Lepidoptera: Pyralidae). Chemosphere 2006, 62, 1388–1393. [Google Scholar] [CrossRef]
- Ravindran, J.; Gupta, N.; Agrawal, M.; Bhaskar, A.B.; Rao, P.L. Modulation of ROS/MAPK signaling pathways by okadaic acid leads to cell death via, mitochondrial mediated caspase-dependent mechanism. Apoptosis 2011, 16, 145–161. [Google Scholar] [CrossRef]
- Chi, C.; Giri, S.S.; Jun, J.W.; Yun, S.; Kim, H.J.; Kim, S.G.; Park, S.C. Immune response of the bay scallop, Argopecten irradians, after exposure to the algicide palmitoleic acid. Fish. Shellfish Immunol. 2016, 57, 371–378. [Google Scholar] [CrossRef]
- Jones, T.O.; Whyte, J.N.C.; Townsend, L.D.; Ginther, N.G.; Iwama, G.K. Effects of domoic acid on haemolymph pH, PCO2 and PO2 in the Pacific oyster, Crassostrea gigas and the California mussel, Mytilus californianus. Aquat. Toxicol. 1995, 31, 43–55. [Google Scholar] [CrossRef]
- Hannam, M.L.; Bamber, S.D.; Moody, A.J.; Galloway, T.S.; Jones, M.B. Immune function in the Arctic Scallop, Chlamys islandica, following dispersed oil exposure. Aquat. Toxicol. 2009, 92, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hannam, M.L.; Bamber, S.D.; Moody, A.J.; Galloway, T.S.; Jones, M.B. Immunotoxicity and oxidative stress in the Arctic scallop Chlamys islandica: Effects of acute oil exposure. Ecotox. Environ. Saf. 2010, 73, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.F.; Proença, L.A.; Barracco, M.A. Comparative study of various immune parameters in three bivalve species during a natural bloom of Dinophysis acuminata in Santa Catarina Island, Brazil. Toxins 2010, 2, 1166–1178. [Google Scholar]
- Simões, E.; Vieira, R.C.; Schramm, M.A.; Mello, D.F.; Pontinha, V.D.A.; Silva, P.M.D.; Barracco, M.A. Impact of harmful algal blooms (Dinophysis acuminata) on the immune system of oysters and mussels from Santa Catarina, Brazil. J. Mar. Biol. Assoc. UK 2015, 95, 773–781. [Google Scholar] [CrossRef]
- Manfrin, C.; Dreos, R.; Battistella, S.; Beran, A. Mediterranean mussel gene expression profile induced by okadaic acid exposure. Environ. Sci. Technol. 2010, 44, 8276–8283. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.L.; Song, L.S.; Song, X.Y.; Wang, B.; Mu, C.K.; Zhang, Y. A fibrinogen-related protein from bay scallop Argopecten irradians involved in innate immunity as pattern recognition receptor. Fish. Shellfish Immunol. 2009, 26, 56–64. [Google Scholar] [CrossRef]
- Ni, D.J.; Song, L.S.; Wu, L.T.; Chang, Y.Q.; Yu, Y.D.; Qiu, L.M.; Wang, L.L. Molecular cloning and mRNA expression of peptidoglycan recognition protein (PGRP) gene in bay scallop (Argopecten irradians, Lamarck 1819). Dev. Comp. Immunol. 2006, 31, 548–558. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Zhang, S.; Zhang, G.F. Cloning, genomic structure, and expression analysis of peroxiredoxin V from bay scallop Argopecten irradians. Fish. Shellfish Immunol. 2011, 30, 309–316. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Miles, A.T.; Hawksworth, G.M.; Beattie, J.; Rodilla, V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. CRC Crit. Rev. Biochem. 2000, 35, 35–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, L.S.; Ni, D.J.; Zhang, H.; Liu, W.Q. Alteration of metallothionein mRNA in bay scallop Argopecten irradians under cadmium exposure and bacteria challenge. Comp. Biochem. Physiol. C 2008, 149, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J. Effect of the algaecide palmitoleic acid on the immune function of the bay scallop Argopecten irradians. Molecules 2016, 21, 610. [Google Scholar] [CrossRef] [PubMed]






| Genes | Primer Sequence | Accession No. |
|---|---|---|
| β-actin | F:5’CAAACAGCAGCCTCCTCGTCA 3’ R: 5’CTGGGCACCTGAACCTTTCGTT 3’ | AY335441 |
| CTL6 | F: 5’CAGTTGCTACAGGGTTCG 3’ R: 5’GGGCGTTATCTGGCTCAT 3’ | GQ202279 |
| FREP | F: 5’CGTCGCAAATGCTGAAGATG 3’ R: 5’TAAGTTGTGGTCGGTCCTGAGA 3’ | EU399719 |
| HSP90 | F: 5’TCAGTATGGTTGGTCCGCTAA 3’ R: 5’CGGTTGCCTTTTCCTTCAGA 3’ | EF532406 |
| MT | F: 5’AACTTGCTGTAGTGGGAATG 3’ R: 5’AGGCTGGAAACTGCTGTGGT 3’ | EU734181 |
| PGRP | F: 5’GGGCAAGTGTATGAGGGAAGAG 3’ R: 5’TCCGATGAAGGAGACAGCGTAG 3’ | AY437875 |
| PrxV | F: 5’AATCAAGGAGCGGCTGGCA 3’ R: 5’TCAACTTCTCAATCTTCCCGTCAT 3’ | HM461987 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.; Zhang, C.; Liu, J.; Zheng, X. Effects of Marine Toxin Domoic Acid on Innate Immune Responses in Bay Scallop Argopecten irradians. J. Mar. Sci. Eng. 2019, 7, 407. https://doi.org/10.3390/jmse7110407
Chi C, Zhang C, Liu J, Zheng X. Effects of Marine Toxin Domoic Acid on Innate Immune Responses in Bay Scallop Argopecten irradians. Journal of Marine Science and Engineering. 2019; 7(11):407. https://doi.org/10.3390/jmse7110407
Chicago/Turabian StyleChi, Cheng, Caiyan Zhang, Jiadai Liu, and Xiaochuan Zheng. 2019. "Effects of Marine Toxin Domoic Acid on Innate Immune Responses in Bay Scallop Argopecten irradians" Journal of Marine Science and Engineering 7, no. 11: 407. https://doi.org/10.3390/jmse7110407
APA StyleChi, C., Zhang, C., Liu, J., & Zheng, X. (2019). Effects of Marine Toxin Domoic Acid on Innate Immune Responses in Bay Scallop Argopecten irradians. Journal of Marine Science and Engineering, 7(11), 407. https://doi.org/10.3390/jmse7110407