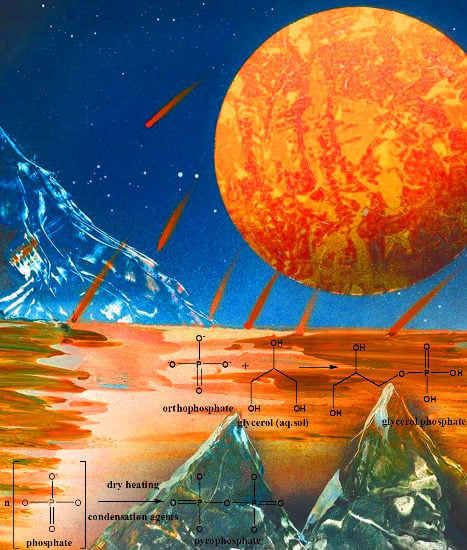
Prebiotic Phosphorylation Reactions on the Early Earth
Abstract
:1. Introduction

2. Biological Significance of Phosphorus

3. Phosphorus Minerals and the Origin of Life
4. Prebiotic Phosphorylation by Phosphates
 R-O-PO32− + H2O [2]
R-O-PO32− + H2O [2]
| Organic | OP Source | Reaction Conditions/Catalysts | Products | Yields | Ref. |
|---|---|---|---|---|---|
| Uridine, thymidine | NaH2PO4 | 65–95 °C, 5–24 h, ethylisocyanide | Uridine & thymidine phosphates | 12% | [20] |
| D-ribose | Sodium phosphate | 25 °C, 21–72 h, cyanogen | Ribofuranose phosphate | 6%–8% | [21] |
| Glucose-1- PO4 or glucose-6- PO4 | Na2HPO4/with or without | Room temperature, 2 h, cyanogen | Glucose-1,6-di-Phosphates | 1%–3% | [22] |
| Fructose | Na2HPO4 | Room temperature, 2 h, cyanogen | Fructose Phosphates | 15% | [23] |
| Thymidine | Apatite | 90 °C, 1 week, heating leading to dryness, ammonium oxalate, cyanides | Thymidine Phosphates | 10%–83% | [24] |
| Uridine | Na2HPO4, Ca(HPO4)2 NaH2PO4 | 160 °C | Uridine Phosphates | 0%–16% | [25] |
| Thymidine | Na2HPO4 | 65 °C, urea | Thymidine Phosphates | 25% | [26] |
| Adenosine | (NH4)2HPO4, KH2PO4 | Electric discharge, cyanate | Adenosine Phosphates | 2% | [27,28] |
| Nucleosides | Na2HPO4 | 100 °C, urea | Nucleoside Phosphates | 18% | [29] |
| Glucose | H3PO4 | Room temperature, dicyanamide | Glucose Phosphate | 1.9% | [30] |
| Uridine | Ca3(PO4)2 | 85 °C, urea | Uridine Phosphates | 30%–80% | [16] |
| Trehalose | NaH2PO4 | 56 °C | Trehalose Phosphates | 15% | [31] |
| Glycerol | NH4H2PO4 | 85 °C, urea | Gycerol Phosphates | 30% | [32] |
| Thymidine and Uridine | NH4H2PO4 | 100 °C, urea | Thymidine and Uridine Phosphates | 33% | [33] |
| Choline Chloride, Glycerol | Struvite, monetite | 75–85 °C | Choline and Glycerol Phosphates | 3%–30% | [17] |
| Uridine | Various OP (H3PO4, NaH2PO4. 2H2O, Na2HPO4. 7H2O, NH4H2PO4, Ca3(PO4)2 etc.) | 2 h, 125–160 °C | Uridine Phosphates | 0%–16% | [34] |
5. Clays, Minerals or Salt Catalyzed Prebiotic Phosphorylations by Using OP
| Organic | OP Source | Reaction Conditions/Catalysts | Products | Yields | Ref. |
|---|---|---|---|---|---|
| Chimyl alcohol *, dodecanoate | NaH2PO4 | 65 °C, cyanamide, kaolin, silicic acid | Phospholipids | 0.015%–0.2% | [39] |
| Glycerol, Ethanolamine | H3PO4 | 100–200 °C hydrothermal conditions, minerals | Glycerol and Ethanolamine Phosphates | 0%–1% | [40] |
| Glucose | H3PO4 | 100–160 °C, hydrothermal conditions, (kaolinite + montmorillonite) | Glucose Phosphates | 0.05%–2% | [41] |
| Uridine | Struvite, Hydroxylapatite | 8–100 °C, few hours 1 week, MgCl2, NH4Cl, urea | Uridine Phosphates | 0%–39% | [42] |
| Uridine, Adenosine, Guanosine, Thymidine | Na2HPO4, Hydroxylapatite (Ca5(PO4)3OH) | 60–100 °C, NH4Cl, urea, NH4HCO3 | Uridine phosphates, Adenosine phosphates, Guanosine phosphates, Thymidine phosphates | 3%–95% | [43] |
6. Phospholipid Synthesis by OP
7. Photochemical Synthesis Reactions of Biological Phosphates
8. Prebiotic Phosphorylation Reactions by Using Condensed/High Energy Phosphates
| Organic | Condensed Phosphate Source | Reaction Conditions/Catalysts | Products | Yields | Ref. |
|---|---|---|---|---|---|
| Glycolaldehyde | Amidotriphosphate | Room temp., 5 days, Mg2+ | Glycolaldehyde phosphate | 76% | [53] |
| Amino acids | Trimetaphosphate (TMP) | 35–45 °C, 16–40 h, pH = 10–11 | N-phosphor-ylated amino acids | 60%–91% | [54] |
| Glyceric acid | TMP | High pH | Phosphoglyceric acid | 40% | [55] |
| Sugars (glyceraldehyde, Ribose, Threose, Erythrose) | Amidotriphosphate or diamidotriphosphate | 4 °C room temperature MgCl2 | Sugar phosphates | 25%–87% | [56] |
| Inosine | Clyclotriphosphate | High pH, room temperature—70 °C | Inosine phosphates | 26%–33% | [57] |
| Adenosine monophosphate | TMP | MgCl2, 100 °C, hydrothermal conditions | Adenosine phosphates | 1% | [58] |
| Beta hydroxyl-n-alkylamines | Cyclic TMP | High pH | Beta hydroxyl-n-alkylamine phosphates | 19%–40% | [59] |
| Nucleosides | TMP | High pH | Nucleotides | 50%–60% | [60] |
| Adenosine | Cyclic TMP | 70–85 °C, Ni2+ | Adenosine phosphates | 30% | [61] |
| (AMP) | Cyclic TMP | Mg2+ heat | Adenosine poly-phosphates | 85%–90% | [62] |
| Adenosine | Graham’s salt, Na4P2O7, Na5P3O10 etc. | Reflux at 100 °C, 4–6 h, high pH | Adenosine phosphates | 0%–1% | [63] |
| Glycolate | TMP | Near-neutral pH , room temperature, few hours 4 months, Hydrotalcite | Phosphoglycolic acid | 34% | [64] |

9. Prebiotic Phosphorylation Reactions in the Alternative Solvents

10. Phosphorylation by Using Reduced Oxidation State Phosphorus Sources
 Fe3O4 + H3PO3 + 5 ½ H2 [90,91]
Fe3O4 + H3PO3 + 5 ½ H2 [90,91]
 [90,91]
[90,91]


11. Conclusions and Recapitulations


Acknowledgments
Conflicts of Interest
References
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef]
- Pasek, M.A.; Kee, T.P. On the Origin of phosphorylated biomolecules. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; pp. 57–84. [Google Scholar]
- Srinivasan, V.; Morowitz, H.J. Analysis of the intermediary metabolism of a reductive chemoautotroph. Biol. Bull. 2009, 217, 222–232. [Google Scholar]
- Pereto, J.; Bada, J.L.; Lazcano, A. Charles Darwin and the origin of life. Origins Life Evol. Biosphere 2009, 39, 395–406. [Google Scholar]
- Westheimer, F.H. Why nature chose phosphate. Science 1987, 235, 1173–1178. [Google Scholar]
- Preiss, J.; Handler, P. Biosynthesis of diphosphopyridine nucleotide. II. Enzymatic aspects. J. Biol. Chem. 1958, 233, 493–500. [Google Scholar]
- Davis, R.D. On the importance of being ionized. Arch. Biochem. Biophys. 1958, 78, 497–509. [Google Scholar] [CrossRef]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Amer. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Brearley, A.J.; Jones, R.H. Chondritic meteorites. Rev. Mineral. Geochem. 1998, 36, 1–398. [Google Scholar]
- Dove, P.M.; Weiner, S. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Arrhenius, G.; Sales, B.; Mojzsis, S.; Lee, T. Entropy and charge in molecular evolution—The case of phosphate. J. Theor. Biol. 1997, 187, 503–522. [Google Scholar] [CrossRef]
- Schwartz, A.W. Phosphorus in prebiotic chemistry. Phil. Trans. Roy. Soc. B—Biol. Sci. 2006, 361, 1743–1749. [Google Scholar] [CrossRef]
- Gulick, A. Phosphorus as a factor in the origin of life. Amer. Sci. 1955, 43, 479–489. [Google Scholar]
- Gedulin, B.; Arrhenius, G. Sources and geochemical evolution of RNA precursor molecules: The role of phosphate. In Early Life on Earth, Nobel Symposium; Bengston, S., Ed.; Columbia University Press: New York, NY, USA, 1994; Volume 84, pp. 91–110. [Google Scholar]
- Pasek, M.A.; Kee, T.P.; Bryant, D.E.; Pavlov, A.A.; Lunine, J.I. Production of potentially prebiotic condensed phosphates by phosphorus redox chemistry. Angew. Chem. Int. Ed. 2008, 47, 7918–7920. [Google Scholar] [CrossRef]
- Handschuh, G.J.; Orgel, L.E. Struvite and prebiotic phosphorylation. Science 1973, 179, 483–484. [Google Scholar]
- Gull, M.; Pasek, M.A. Is struvite a prebiotic mineral? Life 2013, 3, 321–330. [Google Scholar] [CrossRef]
- Pohorille, A.; Pratt, L.R. Is water the universal solvent for life? Origins Life Evol. Biosphere 2012, 42, 405–409. [Google Scholar] [CrossRef]
- Kasting, J.F.; Whitmire, D.P.; Reynolds, R.T. Habitable zones around main sequence stars. Icarus 1993, 101, 108–128. [Google Scholar] [CrossRef]
- Lohrmann, R.; Orgel, L.E. Prebiotic synthesis: Phozsphorylation in aqueous solution. Science 1968, 161, 64–66. [Google Scholar]
- Halmann, M.; Sanchez, R.A.; Orgel, L.E. Phosphorylation of D-ribose in aqueous solution. J. Org. Chem. 1969, 34, 3702–3703. [Google Scholar] [CrossRef]
- Degani, C.; Halmann, M. D-glucose 1-phosphate formation by cyanogen-induced phosphorylation of D-glucose. Synthesis, mechanism, and application to other reducing sugars. J. Chem. Soc. Chem. Commun. 1971, 1459–1465. [Google Scholar] [CrossRef]
- Degani, C.; Kawatsuji, M.; Halmann, M. Cyanogen induced phosphorylation of D-fructose. J. Mol. Evol. 1975, 6, 51–60. [Google Scholar] [CrossRef]
- Schwartz, A.W.; van-der, V.M.; Bisseling, T.; Chittenden, G.J.F. Prebiotic nucleotide synthesis—Demonstration of a geologically plausible pathway. Origins Life Evol. Biosphere 1975, 6, 163–168. [Google Scholar]
- Ponnamperuma, C.; Mack, R. Nucleotide synthesis under possible primitive earth conditions. Science 1965, 148, 1221–1223. [Google Scholar]
- Bishop, M.J.; Lohrman, R.; Orgel, L.E. Prebiotic phosphorylation of thymidine at 65 °C in simulated desert conditions. Nature 1972, 237, 162–164. [Google Scholar] [CrossRef]
- Yamagata, Y.; Matsukawa, T.; Mohri, T.; Inomata, K. Phosphorylation of adenosine in aqueous-solution by electric discharges. Nature 1979, 282, 284–286. [Google Scholar] [CrossRef]
- Yamagata, Y.; Mohri, T.; Yamakoshi, M.; Inomata, K. Constant AMP synthesis in aqueous solution by electric discharges. Origins Life Evol. Biosphere 1981, 11, 233–235. [Google Scholar] [CrossRef]
- Reimann, R.; Zubay, G. Nucleoside phosphorylation: A feasible step in the prebiotic pathway to RNA. Origins Life Evol. Biosphere 1999, 29, 229–247. [Google Scholar] [CrossRef]
- Steinmam, G.; Kenyon, D.H.; Calvin, M. Dehydration condensation in aqueous solution. Nature 1965, 206, 707–708. [Google Scholar] [CrossRef]
- Terelli, E.; Wheeler, S.F. Formation of esters, especially phosphate esters, under “dry” conditions and “mild” pH. Chem. Ind. 1993, 3, 164–165. [Google Scholar]
- Epps, D.E.; Nooner, D.W.; Eichberg, J.; Sherwood, E.; Oró, J. Cyanamide mediated synthesis under plausible primitive earth conditions. VI. The synthesis of glycerol and glycerophosphates. J. Mol. Evol. 1979, 14, 235–241. [Google Scholar] [CrossRef]
- Österberg, R.; Orgel, L.E.; Lohrmann, R. Further studies of urea-catalyzed phosphorylation reactions. J. Mol. Evol. 1973, 2, 231–234. [Google Scholar] [CrossRef]
- Rabinowitz, J.; Chang, S.; Ponnamperuma, C. Phosphorylation by way of inorganic phosphate as a potential prebiotic process. Nature 1968, 218, 442–443. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sverjensky, D.A. Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harbor Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Parks, G.A. Surface energy and adsorption at mineral-water interfaces: An introduction. Rev. Mineral. Geochem. 1990, 23, 133–175. [Google Scholar]
- Davis, J.A.; Kent, D.B. Surface complexation modeling in aqueous geochemistry. Rev. Mineral. Geochem. 1990, 23, 177–260. [Google Scholar]
- Cappellen, P.V.; Charlet, L.; Stumm, W.; Wersin, P. A surface complexation model of the carbonate mineral-aqueous solution interface. Geochim. Cosmochim. Acta 1993, 57, 3505–3518. [Google Scholar] [CrossRef]
- Hargreaves, W.R.; Mulvihill, S.J.; Deamer, D.W. Synthesis of phospholipids and membranes in prebiotic conditions. Nature 1977, 266, 78–80. [Google Scholar] [CrossRef]
- Gull, M.; Ge, T.; Yingwu, W.; Chao, H.; Zhan, S.; Hongming, Y.; Shouhua, F. Resolving the enigma of prebiotic C-O-P bond formation: Prebiotic hydrothermal synthesis of important biological phosphate esters. Heteroat. Chem. 2010, 21, 161–167. [Google Scholar]
- Gull, M.; Yu, W.; Yingwu, W.; Zhan, S.; Ge, T.; Shouhua, F. Mimicking the prebiotic acidic hydrothermal environment: One-pot prebiotic hydrothermal synthesis of glucose phosphates. Heteroat. Chem. 2011, 22, 186–191. [Google Scholar]
- Handschuh, G.J.; Lohrmann, R.; Orgel, L.E. The effect of Mg2+ and Ca2+ on urea-catalyzed phosphorylation reactions. J. Mol. Evol. 1973, 2, 251–262. [Google Scholar] [CrossRef]
- Lohrmann, L.; Orgel, L.E. Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science 1971, 171, 490–494. [Google Scholar]
- Pitsch, S.; Eschenmose, A.; Gedulin, B.; Hui, S.; Arrhenius, G. Mineral induced formation of sugar phosphates. Orig. Life Evol. Biosph. 1995, 25, 297–334. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Pitsch, S.; Arrhenius, G. Mineral induced formation of pentose-2,4-bisphosphates. Origins Life Evol. Biosphere 1999, 29, 139–152. [Google Scholar] [CrossRef]
- Thomas, G.; Maguy, J.; Thomas, O.; Aaron-Albert, H.; France, C-T.; Jean-Francois, L. Inorganic phosphate and nucleotides on silica surface: Condensation, dismutation, and phosphorylation. J. Phys. Chem. 2013, 117, 12579–12590. [Google Scholar]
- Epps, D.E.; Sherwood, E.; Eichberg, J.; Oró, J. Cyanamide mediated syntheses under plausible primitive earth conditions V. The synthesis of phosphatidic acids. J. Mol. Evol. 1978, 11, 279–292. [Google Scholar] [CrossRef]
- Rao, M.; Eichberg, J.; Oró, J. Synthesis of Phosphatidylcholine under possible primitive earth conditions. J. Mol. Evol. 1982, 18, 196–202. [Google Scholar]
- Hagan, W.J. Uracil-catalyzed synthesis of acetyl phosphate: A photochemical driver for protometabolism. Chem. Biol. Chem. 2010, 11, 383–387. [Google Scholar]
- Saygin, O. Nonenzymatic photophosphorylation of acetate by carbamyl phosphate. A model reaction for prebiotic activation of carboxyl groups. Origins Life Evol. Biosphere 1983, 13, 43–48. [Google Scholar]
- Brown, M.R.; Kornberg, A. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. USA 2004, 101, 16085–16087. [Google Scholar] [CrossRef]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 1991, 352, 516–519. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Arrhenius, G.; Eschenmoser, A. Formation of glycolaldehyde phosphate from glycolaldehyde in aqueous solution. Orig. Life Evol. Biosph. 1999, 29, 333–354. [Google Scholar] [CrossRef]
- Feng, N.; Shuting, S.; Chao, H.; Yufen, Zhao. N-phosphorylation of amino acids by using trimetaphosphate. Sphate in aqueous solution-learning from prebiotic synthesis. Green Chem. 2009, 11, 569–573. [Google Scholar] [CrossRef]
- Kolb, V.; Orgel, L.E. Phosphorylation of glyceric acid in aqueous solution using trimetaphosphate. Origins Life Evol. Biosphere 1996, 26, 7–13. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Guntha, S.; Eschenmoser, A. Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed. 2000, 39, 2281–2285. [Google Scholar] [CrossRef]
- Mitsutomo, T.; Mayumi, F.; Shigeru, O. Phosphorylation of inosine with cyclo-triphosphate. Chem. Lett. 1981, 1981, 849–852. [Google Scholar]
- Ozawa, K.; Nemoto, A.; Imai, E-I.; Honda, H.; Hatori, K.; Matsuno, K. Phosphorylation of nucleotide molecules in hydrothermal environments. Origins Life Evol. Biosphere 2004, 34, 465–471. [Google Scholar]
- Mullen, L.B.; Sutherland, J.D. Formation of potentially prebiotic amphiphiles by reaction of beta-hydroxy-n-alkylamines with cyclotriphosphate. Angew. Chem. Int. Ed. 2007, 46, 4166–4168. [Google Scholar] [CrossRef]
- Saffhill, R. Selective phosphorylation of the cis-2',3'-diol of unprotected ribonucleosides with trimetaphosphate in aqueous solution. J. Org. Chem. 1970, 35, 2881–2883. [Google Scholar] [CrossRef]
- Cheng, C.; Fan, C.; Wan, R.; Tong, C.; Miao, Z.; Chen, J.; Zhao, Y. Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Origins Life Evol. Biosphere 2002, 32, 219–224. [Google Scholar] [CrossRef]
- Lohrmann, R. Formation of nucleoside-5'-phosphoramidates under potentially prebiological conditions. J. Mol. Evol. 1977, 10, 137–154. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Ponnamperuma, C. Phosphorylation of adenosine with linear polyphosphate salts in aqueous solution. Nature 1968, 218, 443. [Google Scholar] [CrossRef]
- Kolb, V.; Zhang, S.; Xu, Y.; Arrhenius, G. Mineral induced phosphorylation of glycolate ion—A Metaphor in chemical evolution. Origins Life Evol. Biosphere 1997, 27, 485–503. [Google Scholar] [CrossRef]
- Lindh, U. Biological functions of the elements. In Essentials of Medical Geology; Impacts of the Natural Environments on Public Health; Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., Smedley, P., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 115–160. [Google Scholar]
- Holm, N.G. The significance of Mg in prebiotic Geochemistry. Geobiology 2012, 10, 269–279. [Google Scholar] [CrossRef]
- Yamagata, Y.; Inoue, H.; Inomata, K. Specific effect of magnesium ion on 2',3'-cyclic AMP synthesis from adenosine and trimeta phosphate in aqueous solution. Origins Life Evol. Biosphere 1995, 25, 47–52. [Google Scholar] [CrossRef]
- Prieur, B.E. Phosphorylation of ribose in the presence of borate salts. Origins Life Evol. Biosphere 2009, 39, 264–265. [Google Scholar]
- Scorei, R. Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on earth. Origins Life Evol. Biosphere 2012, 42, 3–17. [Google Scholar] [CrossRef]
- Schoffstall, A.M. Prebiotic phosphorylation of nucleosides in formamide. Origins Life Evol. Biosphere 1976, 7, 399–412. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Mauro, E.D. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, J.S.; Voecks, G.E.; Hobby, G.L.; Ferris, J.P.; Williams, E.A.; Nicodem, D.E. Ultraviolet-gas phase and -photocatalytic synthesis from CO and NH3. J. Mol. Evol. 1975, 5, 223–241. [Google Scholar] [CrossRef]
- Marsh, J.D.F.; Martin, M.J. The hydrolysis and polymerization of hydrogen cyanide in alkaline solutions. J. Appl. Chem. 1957, 7, 205–209. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in prebiotic synthesis. II. Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J. Mol. Biol. 1967, 30, 223–253. [Google Scholar]
- Solomon, P.M. Interstellar molecules. Phys. Today 1973, 26, 32–40. [Google Scholar]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; Mauro, E.D. Nucleoside phosphorylation by phosphate minerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar]
- Schoffstall, A.M.; Barto, R.J.; Ramos, D.L. Nucleoside and deoxynucleoside phosphorylation in formamide solutions. Origins Life Evol. Biosphere 1982, 12, 143–151. [Google Scholar] [CrossRef]
- Schoffstall, A.M.; Laing, E.M. Phosphorylation mechanisms in chemical evolution. Orig. Life Evol. Biosph. 1985, 15, 141–150. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, G.; Pino, S.; Costanzo, G.; di Mauro, E. Genetics first or metabolism first? The formamide clue. Chem. Soc. Rev. 2012, 41, 5526–5565. [Google Scholar]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic phosphate ester syntheses in a deep eutectic solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Mamajanov, I.; Engelhart, A.E.; Bean, H.D.; Hud, N.V. DNA and RNA in anhydrous media: Duplex, triplex, and g-quadruplex secondary structures in a deep eutectic solvent. Angew. Chem. Int. Ed. 2010, 49, 6310–6314. [Google Scholar] [CrossRef]
- Austin, S.M.; Waddell, T.G. Prebiotic synthesis of vitamin B6-type compounds. Origins Life Evol. Biosphere 1999, 29, 287–296. [Google Scholar] [CrossRef]
- Maugeri, Z.; Leitner, W.; de Dominguez, M.P. Chymotrypsin-catalyzed peptide synthesis in deep eutectic solvents. Eur. J. Org. Chem. 2013, 2013, 4223–4228. [Google Scholar]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; Agostino, C.D.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green. Chem. 2011, 13, 82–90. [Google Scholar]
- Cooper, G.W.; Onwo, W.M.; Cronin, J.R. Alkyl phosphonic-acids and sulfonic-acids in the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4109–4115. [Google Scholar] [CrossRef]
- Graaf, R.M.D.; Visscher, J.; Schwartz, A.W. A plausibly prebiotic synthesis of phosphonic acids. Nature 1995, 378, 474–477. [Google Scholar] [CrossRef]
- Graaf, R.M.D.; Schwartz, A.W. Reduction and activation of phosphate on the primitive earth. Origins Life Evol. Biosphere 2000, 30, 405–410. [Google Scholar] [CrossRef]
- Glindemann, D.; Graaf, R.M.D.; Schwartz, A.W. Chemical reduction of phosphate on the primitive Earth. Origins Life Evol. Biosphere 1999, 29, 555–561. [Google Scholar] [CrossRef]
- Graaf, R.M.D.; Schwartz, A.W. Thermal synthesis of nucleoside H-phosphonates under mild conditions. Origins Life Evol. Biosphere 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Pasek, M.A.; Lauretta, D.S. Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 2005, 5, 515–535. [Google Scholar] [CrossRef]
- Pasek, M.A.; Dworkin, J.P.; Lauretta, D.S. A radical pathway for organic phosphorylation during schreibersite corrosion with implications for the origin of life. Geochim. Cosmochim. Acta 2007, 71, 1721–1736. [Google Scholar] [CrossRef]
- Bryant, D.E.; Kee, T.P. Direct evidence for the availability of reactive, water soluble phosphorus on the early earth. H-phosphinic acid from the Nantan meteorite. Chem. Commun. 2006, 22, 2344–2346. [Google Scholar] [CrossRef]
- Gorrell, I.B.; Wang, L.; Marks, A.J.; Bryant, D.E.; Bouillot, F.; Goddard, A.; Heard, D.E.; Kee, T.P. On the origin of Murchison meteorite phosphonates. Implications for prebiotic chemistry. Chem. Commun. 2006, 21, 1643–1645. [Google Scholar]
- Bryant, D.E.; Marriott, K.E.R.; Macgregor, S.A.; Kilner, C.; Pasek, M.A.; Kee, T.P. On the prebiotic potential of reduced oxidation state phosphorus: The H-phosphinate-pyruvate system. Chem. Commun. 2010, 46, 3726–3728. [Google Scholar] [CrossRef]
- Bryant, D.E.; Greenfield, D.; Walshaw, R.D.; Evans, S.M.; Nimmo, A.E.; Smith, C.; Wang, L.; Pasek, M.A.; Kee, T.P. Electrochemical studies of iron meteorites. Phosphorus redox chemistry on the early earth. Int. J. Astrobiol. 2009, 8, 27–36. [Google Scholar] [CrossRef]
- Kee, T.P.; Bryant, D.E.; Herschy, B.; Marriott, K.E.R.; Cosgrove, N.E.; Pasek, M.A.; Atlas, Z.D.; Cousins, C.R. Phosphate activation via reduced oxidation state phosphorus (P). Mild routes to condensed-P energy currency molecules. Life 2013, 3, 386–402. [Google Scholar] [CrossRef] [Green Version]
- Pasek, M.A.; Harnmeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early archean ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 10089–10094. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gull, M. Prebiotic Phosphorylation Reactions on the Early Earth. Challenges 2014, 5, 193-212. https://doi.org/10.3390/challe5020193
Gull M. Prebiotic Phosphorylation Reactions on the Early Earth. Challenges. 2014; 5(2):193-212. https://doi.org/10.3390/challe5020193
Chicago/Turabian StyleGull, Maheen. 2014. "Prebiotic Phosphorylation Reactions on the Early Earth" Challenges 5, no. 2: 193-212. https://doi.org/10.3390/challe5020193
APA StyleGull, M. (2014). Prebiotic Phosphorylation Reactions on the Early Earth. Challenges, 5(2), 193-212. https://doi.org/10.3390/challe5020193