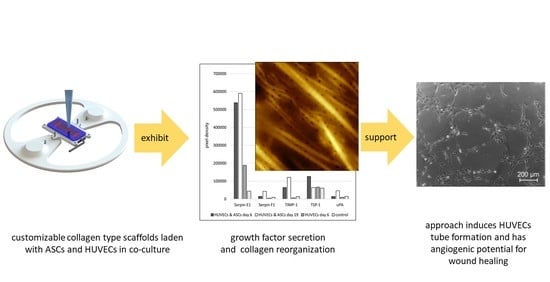
Angiogenic Potential of Co-Cultured Human Umbilical Vein Endothelial Cells and Adipose Stromal Cells in Customizable 3D Engineered Collagen Sheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Human Umbilical Vein Endothelial Cells (HUVECs)
2.2. Cultivation of Adipose Stromal Cells (ASCs)
2.3. Analysis of Proliferation of 2D Co-Culture of ASCs and HUVECs
2.4. 3D Cell Culture in Collagen Sheets
2.5. Preparation of 3D Collagen Sheets Using CAD and 3D-Printing
2.6. Scanning Electron Microscopy (SEM)
2.7. Sample Preparation for Atomic Force Microscopy (AFM)
2.8. AFM Measurements
2.9. AFM Data Analyses
2.10. Angiogenesis Array
2.11. Tube Formation Assay
3. Results
3.1. Proliferation of 2D Co-Culture of ASCs and HUVECs
3.2. Restructuring of Collagen Sheets with HUVECs and ASCs Using Scanning Electron Microscopy (SEM)
3.3. Atomic Force Microscopy (AFM) of Collagen Sheets
3.4. Angiogenic Potential of the 3D Collagen Sheets
3.4.1. Angiogenic Array of the Cell Culture Supernatant Collected from Cell-Laden 3D Collagen Sheets
3.4.2. HUVECs Tube Formation Assay of the Cell Culture Supernatant Obtained from Co-Culture Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreml, S.; Szeimies, R.-M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.K.L.; Fernandes, B.L.; de Souza, M.A. Autologous matrix of platelet-rich fibrin in wound care settings: A systematic review of randomized clinical trials. J. Funct. Biomater. 2020, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.; Chin, G.; Moldawer, L.; Diegelmann, R. Ch23 Mechanisms of Vascular Disease; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Arnold, F.; West, D.C. Angiogenesis in wound healing. Pharmacol. Ther. 1991, 52, 407–422. [Google Scholar] [CrossRef]
- Um, J.; Yu, J.; Dubon, M.J.; Park, K.-S. Substance P and thiorphan synergically enhance angiogenesis in wound healing. Tissue Eng. Regen. Med. 2016, 13, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.S. The feasibility of using pulsatile electromagnetic fields (PEMFs) to enhance the regenerative ability of dermal biomaterial scaffolds. J. Funct. Biomater. 2018, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Bhadada, S.V.; Goyal, B.R.; Patel, M.M. Angiogenic targets for potential disorders. Fundam. Clin. Pharmacol. 2011, 25, 29–47. [Google Scholar] [CrossRef]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef] [Green Version]
- Nissen, N.N.; Polverini, P.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445. [Google Scholar]
- Clark, R.A. The Molecular and Cellular Biology of Wound Repair; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoo, J.J.; Lee, S.J. Three-dimensional cell-based bioprinting for soft tissue regeneration. Tissue Eng. Regen. Med. 2016, 13, 647–662. [Google Scholar] [CrossRef]
- Iyer, K.; Chen, Z.; Ganapa, T.; Wu, B.M.; Tawil, B.; Linsley, C.S. Keratinocyte migration in a three-dimensional in vitro wound healing model co-cultured with fibroblasts. Tissue Eng. Regen. Med. 2018, 15, 721–733. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D photo-fabrication for tissue engineering and drug delivery. Engineering 2015, 1, 090–112. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Kyu-Cheol, N.; Wang, Z.; Cui, Y.; Liu, H.; Liu, H.; Feng, Y.; Zhao, Y.; Lin, Q.; Li, Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020, 11, 2041731420947242. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef] [Green Version]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as bioink for bioprinting: A comprehensive review. Int. J. Bioprint. 2020, 6, 270. [Google Scholar]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Kubota, Y.; Kleinman, H.K.; Martin, G.R.; Lawley, T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.S.; Kinsella, J.L.; Fridman, R.; Auerbach, R.; Piasecki, B.A.; Yamada, Y.; Zain, M.; Kleinman, H.K. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J. Cell. Physiol. 1992, 153, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.A.; Gomes, E.D.; Afonso, J.L.; Granja, S.; Baltazar, F.; Silva, N.A.; Shoichet, M.S.; Sousa, R.A.; Learmonth, D.A.; Salgado, A.J. In vitro Evaluation of ASCs and HUVECs Co-cultures in 3D Biodegradable Hydrogels on Neurite Outgrowth and Vascular Organization. Front. Cell Dev. Biol. 2020, 8, 489. [Google Scholar] [CrossRef] [PubMed]
- Prein, C.; Warmbold, N.; Farkas, Z.; Schieker, M.; Aszodi, A.; Clausen-Schaumann, H. Structural and mechanical properties of the proliferative zone of the developing murine growth plate cartilage assessed by atomic force microscopy. Matrix Biol. 2016, 50, 1–15. [Google Scholar] [CrossRef]
- Muschter, D.; Fleischhauer, L.; Taheri, S.; Schilling, A.F.; Clausen-Schaumann, H.; Grässel, S. Sensory neuropeptides are required for bone and cartilage homeostasis in a murine destabilization-induced osteoarthritis model. Bone 2020, 133, 115181. [Google Scholar] [CrossRef]
- Li, P.; Fleischhauer, L.; Nicolae, C.; Prein, C.; Farkas, Z.; Saller, M.M.; Prall, W.C.; Wagener, R.; Heilig, J.; Niehoff, A. Mice lacking the matrilin family of extracellular matrix proteins develop mild skeletal abnormalities and are susceptible to age-associated osteoarthritis. Int. J. Mol. Sci. 2020, 21, 666. [Google Scholar] [CrossRef] [Green Version]
- Reuten, R.; Zendehroud, S.; Nicolau, M.; Fleischhauer, L.; Laitala, A.; Kiderlen, S.; Nikodemus, D.; Wullkopf, L.; Nielsen, S.R.; McNeilly, S. Basement membrane stiffness determines metastases formation. Nat. Mater. 2021, 20, 892–903. [Google Scholar] [CrossRef]
- Butt, H.-J.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1–7. [Google Scholar] [CrossRef]
- Chandran, P.L.; Dimitriadis, E.K.; Mertz, E.L.; Horkay, F. Microscale mapping of extracellular matrix elasticity of mouse joint cartilage: An approach to extracting bulk elasticity of soft matter with surface roughness. Soft Matter 2018, 14, 2879–2892. [Google Scholar] [CrossRef]
- Rico, F.; Roca-Cusachs, P.; Gavara, N.; Farré, R.; Rotger, M.; Navajas, D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys. Rev. E 2005, 72, 021914. [Google Scholar] [CrossRef] [Green Version]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic colorimetric proliferation assays: MTT, WST, and resazurin. In Cell Viability Assays; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–17. [Google Scholar]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [Green Version]
- Gullberg, D.; Paraskevi, H.; Schaefer, L.; Tenni, R.; Theocharis, A.; Winberg, J.-O. Extracellular Matrix: Pathobiology and Signaling; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-β signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J. Investig. Derm. 2002, 118, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Hochstein, A.O.; Bhatia, A. Collagen: Its role in wound healing. Wound Manag. 2014, 4, 104–109. [Google Scholar]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75, 12–26. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2019; pp. 136–146. [Google Scholar]
- Gomes, S.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Natural and genetically engineered proteins for tissue engineering. Prog. Polym. Sci. 2012, 37, 1–17. [Google Scholar] [CrossRef]
- Ramanathan, G.; Singaravelu, S.; Muthukumar, T.; Thyagarajan, S.; Perumal, P.T.; Sivagnanam, U.T. Design and characterization of 3D hybrid collagen matrixes as a dermal substitute in skin tissue engineering. Mater. Sci. Eng. C 2017, 72, 359–370. [Google Scholar] [CrossRef]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogoşanu, G.D.; Bălşeanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoantă, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2019, 557, 199–207. [Google Scholar] [CrossRef]
- Matsuda, K.; Falkenberg, K.J.; Woods, A.A.; Choi, Y.S.; Morrison, W.A.; Dilley, R.J. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng. Part A 2013, 19, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simone, T.M.; Higgins, C.E.; Czekay, R.-P.; Law, B.K.; Higgins, S.P.; Archambeault, J.; Kutz, S.M.; Higgins, P.J. SERPINE1: A molecular switch in the proliferation-migration dichotomy in wound-“activated” keratinocytes. Adv. Wound Care 2014, 3, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawler, P.R.; Lawler, J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and-2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef] [PubMed]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. JoVE 2014, 1, e51312. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nessbach, P.; Schwarz, S.; Becke, T.D.; Clausen-Schaumann, H.; Machens, H.-G.; Sudhop, S. Angiogenic Potential of Co-Cultured Human Umbilical Vein Endothelial Cells and Adipose Stromal Cells in Customizable 3D Engineered Collagen Sheets. J. Funct. Biomater. 2022, 13, 107. https://doi.org/10.3390/jfb13030107
Nessbach P, Schwarz S, Becke TD, Clausen-Schaumann H, Machens H-G, Sudhop S. Angiogenic Potential of Co-Cultured Human Umbilical Vein Endothelial Cells and Adipose Stromal Cells in Customizable 3D Engineered Collagen Sheets. Journal of Functional Biomaterials. 2022; 13(3):107. https://doi.org/10.3390/jfb13030107
Chicago/Turabian StyleNessbach, Philipp, Sascha Schwarz, Tanja D. Becke, Hauke Clausen-Schaumann, Hans-Guenther Machens, and Stefanie Sudhop. 2022. "Angiogenic Potential of Co-Cultured Human Umbilical Vein Endothelial Cells and Adipose Stromal Cells in Customizable 3D Engineered Collagen Sheets" Journal of Functional Biomaterials 13, no. 3: 107. https://doi.org/10.3390/jfb13030107
APA StyleNessbach, P., Schwarz, S., Becke, T. D., Clausen-Schaumann, H., Machens, H. -G., & Sudhop, S. (2022). Angiogenic Potential of Co-Cultured Human Umbilical Vein Endothelial Cells and Adipose Stromal Cells in Customizable 3D Engineered Collagen Sheets. Journal of Functional Biomaterials, 13(3), 107. https://doi.org/10.3390/jfb13030107