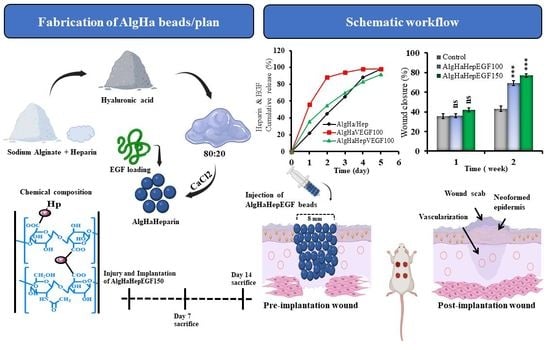
In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing
Abstract
:1. Introduction
2. Material and Methods
2.1. Fabrication of Crosslinked AlgHA Composite Beads
2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.3. Bead Size Distribution and Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Beads Weight Loss
2.5. EGF Release Study
2.6. Biocompatibility
2.7. In Vitro Wound-Healing Migration Assay
2.8. Immunoblotting
2.9. Animal Models and Surgical Procedures
2.10. Histology
3. Statistical Analysis
4. Results
4.1. Characterization of AlgHA-Heparin Beads
4.2. EGF Loading, Entrapment, and Cumulative Release
4.3. MTT Assay and Proliferation
4.4. Effect of EGF on the Migration of L929 Cells
4.5. Western Blot Analysis
4.6. Wound Closure
4.7. Post-Implantation Histological Analysis
4.8. Immunohistochemistry
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, S.E.; Netto, M.; Ambrósio, R., Jr. Corneal cells: Chatty in development, homeostasis, wound healing, and disease. Am. J. Ophthalmol. 2003, 136, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Eaglstein, W.H.; Mertz, P.M. New method for assessing epidermal wound healing: The effects of triamcinolone acetonide and polyethelene film occlusion. J. Investig. Dermatol. 1978, 71, 382–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, J.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Riha, S.M.; Maarof, M.; Fauzi, M.B. Synergistic effect of biomaterial and stem cell for skin tissue engineering in cutaneous wound healing: A concise review. Polymers 2021, 13, 1546. [Google Scholar] [CrossRef]
- Ali, M.; Payne, S.L. Biomaterial-based cell delivery strategies to promote liver regeneration. Biomater. Res. 2021, 25, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, T.; Tengattini, S.; Rezwan, R.; Chiesa, E.; Temporini, C.; Dorati, R.; Massolini, G.; Conti, B.; Ubiali, D.; Terreni, M. Design of epidermal growth factor immobilization on 3D biocompatible scaffolds to promote tissue repair and regeneration. Sci. Rep. 2021, 11, 2629. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef]
- Mikołajczyk, T.; Wołowska-Czapnik, D. Multifunctional alginate fibres with anti-bacterial properties. Fibres Text. East. Eur. 2005, 13, 35–40. [Google Scholar]
- De Vos, P.; De Haan, B.; Van Schilfgaarde, R. Effect of the alginate composition on the biocompatibility of alginate-polylysine microcapsules. Biomaterials 1997, 18, 273–278. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Ren, Y.; Wang, P.; Pu, Y.; Yang, R.; Wang, X.; Tan, X.; Ye, Z.; Maurizot, V. Mussel-inspired dual-cross-linking hyaluronic acid/ε-polylysine hydrogel with self-healing and antibacterial properties for wound healing. ACS Appl. Mater. Interfaces 2020, 12, 27876–27888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly (Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Yeo, K.; Berse, B.; Yeo, T.-K.; Senger, D.R.; Dvorak, H.F.; Van De Water, L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: Different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992, 119, 45–54. [Google Scholar] [CrossRef]
- Niswander, L.; Martin, G.R. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 1992, 114, 755–768. [Google Scholar] [CrossRef]
- Costantino, H.R.; Langer, R.; Klibanov, A.M. Solid-phase aggregation of proteins under pharmaceutically relevant conditions. J. Pharm. Sci. 1994, 83, 1662–1669. [Google Scholar] [CrossRef]
- Ogiwara, K.; Nagaoka, M.; Cho, C.-S.; Akaike, T. Construction of a novel extracellular matrix using a new genetically engineered epidermal growth factor fused to IgG-Fc. Biotechnol. Lett. 2005, 27, 1633–1637. [Google Scholar] [CrossRef]
- Hou, K.; Zaniewski, R.; Roy, S. Protein A immobilized affinity cartridge for immunoglobulin purification. Biotechnol. Appl. Biochem. 1991, 13, 257–268. [Google Scholar] [PubMed]
- Kim, J.K.; Kim, H.J.; Chung, J.-Y.; Lee, J.-H.; Young, S.-B.; Kim, Y.-H. Natural and synthetic biomaterials for controlled drug delivery. Arch. Pharmacal Res. 2014, 37, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Kumar, Y.; Ravindranath, S.S. Thermal degradation of SBS in bitumen during storage: Influence of temperature, SBS concentration, polymer type and base bitumen. Polym. Degrad. Stab. 2018, 147, 64–75. [Google Scholar] [CrossRef]
- Ali, M.; Kwak, S.H.; Lee, B.-T.; Choi, H.J. Controlled release of vascular endothelial growth factor (VEGF) in alginate and hyaluronic acid (ALG–HA) bead system to promote wound healing in punch-induced wound rat model. J. Biomater. Sci. Polym. Ed. 2022, 34, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Samandari, M.; Endo, Y.; Zhang, Y.; Quint, J.; Schmidt, T.A.; Tamayol, A.; Sinha, I. In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact. Mater. 2022, 8, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022, 5, 1111–1125. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; Elichiry-Ortiz, P.T.; Hol, K.; Troost, F.J.; Masclee, A.A.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]
- Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.G.; Bedzinski, R. Analysis of the degradation process of alginate-based hydrogels in artificial urine for use as a bioresorbable material in the treatment of urethral injuries. Processes 2020, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Moya, M.L.; Cheng, M.-H.; Huang, J.-J.; Francis-Sedlak, M.E.; Kao, S.-w.; Opara, E.C.; Brey, E.M. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials 2010, 31, 2816–2826. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Yang, C.-H.; Yen, C.-C.; Jheng, J.-J.; Wang, C.-Y.; Chen, S.-S.; Huang, P.-Y.; Huang, K.-S.; Shaw, J.-F. Immobilization of Brassica oleracea chlorophyllase 1 (BoCLH1) and Candida rugosa lipase (CRL) in magnetic alginate beads: An enzymatic evaluation in the corresponding proteins. Molecules 2014, 19, 11800–11815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirian, J.; Makkar, P.; Lee, G.H.; Paul, K.; Lee, B.T. Incorporation of alginate-hyaluronic acid microbeads in injectable calcium phosphate cement for improved bone regeneration. Mater. Lett. 2020, 272, 127830. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Shoichet, M.S. Modulated protein delivery to engineer tissue repair. Tissue Eng. Part A 2019, 25, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Tae, G.; Scatena, M.; Stayton, P.S.; Hoffman, A.S. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J. Biomater. Sci. Polym. Ed. 2006, 17, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Matsuda, Y.; Takeda, A.; Uchinuma, E.; Kuroyanagi, Y. Effect of EGF and bFGF on fibroblast proliferation and angiogenic cytokine production from cultured dermal substitutes. J. Biomater. Sci. Polym. Ed. 2012, 23, 1315–1324. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, L.J.; Kim, M.J.; Kim, J.-H.; Chung, D.J. In situ sodium alginate-hyaluronic acid hydrogel coating method for clinical applications. Macromol. Res. 2014, 22, 240–247. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, X.; Nune, K.C.; Misra, R. Biodegradable hydrogel-based biomaterials with high absorbent properties for non-adherent wound dressing. Int. Wound J. 2017, 14, 1076–1087. [Google Scholar] [CrossRef]
- Wei, S.; Wang, W.; Li, L.; Meng, H.-Y.; Feng, C.-Z.; Dong, Y.-Y.; Fang, X.-C.; Dong, Q.-Q.; Jiang, W.; Xin, H.-L. Recombinant human epidermal growth factor combined with vacuum sealing drainage for wound healing in Bama pigs. Mil. Med. Res. 2021, 8, 18. [Google Scholar] [CrossRef]
- Lauand, C.; Rezende-Teixeira, P.; Cortez, B.A.; Niero, E.L.d.O.; Machado-Santelli, G.M. Independent of ErbB1 gene copy number, EGF stimulates migration but is not associated with cell proliferation in non-small cell lung cancer. Cancer Cell Int. 2013, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Shamel, M.; Mansy, M.; Mubarak, R. The Effect of EGF on VEGF Expression on Submandibular Salivary Gland of Albino Rats Receiving Doxorubicin. Egypt. J. Histol. 2020. [Google Scholar] [CrossRef]
- Nicolas, S.; Abdellatef, S.; Haddad, M.A.; Fakhoury, I.; El-Sibai, M. Hypoxia and EGF stimulation regulate VEGF expression in human glioblastoma multiforme (GBM) cells by differential regulation of the PI3K/Rho-GTPase and MAPK pathways. Cells 2019, 8, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RAVINDRANATH, N.; WION, D.; BRACHET, P.; DJAKIEW, D. Epidermal growth factor modulates the expression of vascular endothelial growth factor in the human prostate. J. Androl. 2001, 22, 432–443. [Google Scholar]
- van Cruijsen, H.; Giaccone, G.; Hoekman, K. Epidermal growth factor receptor and angiogenesis: Opportunities for combined anticancer strategies. Int. J. Cancer 2005, 117, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Gualano, R.C.; Hibbs, M.L.; Anderson, G.P.; Bozinovski, S. Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J. Biol. Chem. 2008, 283, 9977–9985. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yang, W.; Aldape, K.; He, J.; Lu, Z. Epidermal growth factor (EGF)-enhanced vascular cell adhesion molecule-1 (VCAM-1) expression promotes macrophage and glioblastoma cell interaction and tumor cell invasion. J. Biol. Chem. 2014, 289, 18667. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Jin, D.; Qu, X.; Liu, H.; Chen, X.; Yin, M.; Liu, C. A PEG-Lysozyme hydrogel harvests multiple functions as a fit-to-shape tissue sealant for internal-use of body. Biomaterials 2019, 192, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Min, D.; Guo, G.; Liao, X.; Fu, Z. Experimental study of epidermal growth factor and acidic fibroblast growth factor in the treatment of diabetic foot wounds. Exp. Ther. Med. 2018, 15, 5365–5370. [Google Scholar] [CrossRef] [Green Version]
- Nagaoka, T.; Kaburagi, Y.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Steeber, D.A.; Tedder, T.F.; Sato, S. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am. J. Pathol. 2000, 157, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Hu, Z.; Cao, X.; Huang, S.; Dong, Y.; Cheng, P.; Xu, H.; Shu, B.; Xie, J.; Wu, J. Fibronectin precoating wound bed enhances the therapeutic effects of autologous epidermal basal cell suspension for full-thickness wounds by improving epidermal stem cells’ utilization. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Beyranvand, F.; Gharzi, A.; Abbaszadeh, A.; Khorramabadi, R.M.; Gholami, M.; Gharravi, A.M. Encapsulation of Satureja khuzistanica extract in alginate hydrogel accelerate wound healing in adult male rats. Inflamm. Regen. 2019, 39, 2. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Song, Y.; Weir, M.D.; Sun, J.; Zhao, L.; Simon, C.G.; Xu, H.H. A self-setting iPSMSC-alginate-calcium phosphate paste for bone tissue engineering. Dent. Mater. 2016, 32, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Amirian, J.; Van, T.T.T.; Bae, S.-H.; Jung, H.-I.; Choi, H.-J.; Cho, H.-D.; Lee, B.-T. Examination of in vitro and in vivo biocompatibility of alginate-hyaluronic acid microbeads as a promising method in cell delivery for kidney regeneration. Int. J. Biol. Macromol. 2017, 105, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Taz, M.; Makkar, P.; Imran, K.M.; Jang, D.; Kim, Y.-S.; Lee, B.-T. Bone regeneration of multichannel biphasic calcium phosphate granules supplemented with hyaluronic acid. Mater. Sci. Eng. C 2019, 99, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Davison, N.L.; Barrère-de Groot, F.; Grijpma, D.W. Degradation of biomaterials. Tissue Eng. 2014, 177–215. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Oino, D.; Ohira, H.; Muguruma, H.; Moczko, E.; Piletsky, S.A. Size of heparin-imprinted nanoparticles reflects the matched interactions with the target molecule. Sensors 2019, 19, 2415. [Google Scholar] [CrossRef] [Green Version]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Theocharis, A.D.; Pavão, M.S.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key players in cancer cell biology and treatment. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef]
- Skop, N.B.; Calderon, F.; Levison, S.W.; Gandhi, C.D.; Cho, C.H. Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomater. 2013, 9, 6834–6843. [Google Scholar] [CrossRef]
- Tanihara, M.; Suzuki, Y.; Yamamoto, E.; Noguchi, A.; Mizushima, Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J. Biomed. Mater. Res. 2001, 56, 216–221. [Google Scholar] [CrossRef]
- Sun, B.; Chen, B.; Zhao, Y.; Sun, W.; Chen, K.; Zhang, J.; Wei, Z.; Xiao, Z.; Dai, J. Crosslinking heparin to collagen scaffolds for the delivery of human platelet-derived growth factor. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Baldwin, A.; Yamaguchi, N.; Kiick, K.L. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J. Control. Release 2007, 122, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Peplow, P.V. Glycosaminoglycan: A candidate to stimulate the repair of chronic wounds. Thromb. Haemost. 2005, 94, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Greer, I.A. The potential role of heparin in assisted conception. Hum. Reprod. Update 2008, 14, 623–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greinacher, A. Heparin-induced thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.R.; Hull, R.D.; Brant, R.; Hogan, D.B.; Pineo, G.F.; Raskob, G.E. Aging and heparin-related bleeding. Arch. Intern. Med. 1996, 156, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Yahata, Y.; Shirakata, Y.; Tokumaru, S.; Yang, L.; Dai, X.; Tohyama, M.; Tsuda, T.; Sayama, K.; Iwai, M.; Horiuchi, M. A novel function of angiotensin II in skin wound healing: Induction of fibroblast and keratinocyte migration by angiotensin II via heparin-binding epidermal growth factor (EGF)-like growth factor-mediated EGF receptor transactivation. J. Biol. Chem. 2006, 281, 13209–13216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsh, J. Heparin. N. Engl. J. Med. 1991, 324, 1565–1574. [Google Scholar]
- Choi, W.I.; Sahu, A.; Vilos, C.; Kamaly, N.; Jo, S.-M.; Lee, J.H.; Tae, G. Bioinspired heparin nanosponge prepared by photo-crosslinking for controlled release of growth factors. Sci. Rep. 2017, 7, 14351. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, A.; Bhanu, S. Dynamics of controlled release of heparin from swellable crosslinked starch microspheres. J. Mater. Sci. Mater. Med. 2007, 18, 1613–1621. [Google Scholar] [CrossRef]
- Cribbs, R.; Luquette, M.; Besner, G.E. Acceleration of partial-thickness burn wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF). J. Burn. Care Rehabil. 1998, 19, 95–101. [Google Scholar] [CrossRef]
- Ji, C.; Cao, C.; Lu, S.; Kivlin, R.; Amaral, A.; Kouttab, N.; Yang, H.; Chu, W.; Bi, Z.; Di, W. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008, 62, 857–865. [Google Scholar] [CrossRef]
- Dogan, S.; Demirer, S.; Kepenekci, I.; Erkek, B.; Kiziltay, A.; Hasirci, N.; Müftüoğlu, S.; Nazikoğlu, A.; Renda, N.; Dincer, U. Epidermal growth factor-containing wound closure enhances wound healing in non-diabetic and diabetic rats. Int. Wound J. 2009, 6, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Messersmith, W.A. New strategies in colorectal cancer: Biomarkers of response to epidermal growth factor receptor monoclonal antibodies and potential therapeutic targets in phosphoinositide 3-kinase and mitogen-activated protein kinase pathways. Clin. Cancer Res. 2010, 16, 3811–3818. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-J.; Xiao, M.; Balint, K.; Smalley, K.S.; Brafford, P.; Qiu, R.; Pinnix, C.C.; Li, X.; Herlyn, M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006, 66, 4182–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Z.; Wang, J.; Guo, Q.; Fu, Y.; Dai, Z.; Wang, M.; Bai, Y.; Liu, X.; Cooper, P.R. Amphiregulin regulates odontogenic differentiation of dental pulp stem cells by activation of mitogen-activated protein kinase and the phosphatidylinositol 3-kinase signaling pathways. Stem Cell Res. Ther. 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.L.; Curtsinger, L., III; Brightwell, J.R.; Ackerman, D.M.; Tobin, G.R.; Polk Jr, H.C.; George-Nascimento, C.; Valenzuela, P.; Schultz, G.S. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J. Exp. Med. 1986, 163, 1319–1324. [Google Scholar] [CrossRef]
- Goh, M.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Patel, M.; Velagapudi, C.; Burns, H.; Doss, R.; Lee, M.-J.; Mariappan, M.M.; Wagner, B.; Arar, M.; Barnes, V.L.; Abboud, H.E. Mouse Metanephric Mesenchymal Cell–Derived Angioblasts Undergo Vasculogenesis in Three-Dimensional Culture. Am. J. Pathol. 2018, 188, 768–784. [Google Scholar] [CrossRef] [Green Version]
- Dustin, M.L.; Rothlein, R.; Bhan, A.K.; Dinarello, C.A.; Springer, T.A. Induction by IL 1 and interferon-gamma: Tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J. Immunol. 1986, 137, 245–254. [Google Scholar] [CrossRef]
- Middleton, M.H.; Norris, D.A. Cytokine-induced ICAM-1 expression in human keratinocytes is highly variable in keratinocyte strains from different donors. J. Investig. Dermatol. 1995, 104, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Qin, L.; Chen, C.; Hu, Q.; Wang, J.; Shen, J. Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol. Int. 2021, 45, 1976–1985. [Google Scholar] [CrossRef]
- Carpenter, G.; Cohen, S. Human epidermal growth factor and the proliferation of human fibroblasts. J. Cell. Physiol. 1976, 88, 227–237. [Google Scholar] [CrossRef]
- Pruss, R.M.; Herschman, H.R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc. Natl. Acad. Sci. USA 1977, 74, 3918–3921. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.; Cohen, S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J. Cell Biol. 1976, 71, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Wiley, H.S.; Cunningham, D.D. A steady state model for analyzing the cellular binding, internalization and degradation of polypeptide ligands. Cell 1981, 25, 433–440. [Google Scholar] [CrossRef] [PubMed]









| Beads Type | Alginate (2%) (w/v) | Hyaluronic Acid (2%) (w/v) | Heparin |
|---|---|---|---|
| AlgHAHep | 80 mL | 20 mL | 5 IU/ml |
| AlgHA | 80 ml | 20 ml | No heparin added |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.; Kwak, S.H.; Byeon, J.Y.; Choi, H.J. In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing. J. Funct. Biomater. 2023, 14, 403. https://doi.org/10.3390/jfb14080403
Ali M, Kwak SH, Byeon JY, Choi HJ. In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing. Journal of Functional Biomaterials. 2023; 14(8):403. https://doi.org/10.3390/jfb14080403
Chicago/Turabian StyleAli, Maqsood, Si Hyun Kwak, Je Yeon Byeon, and Hwan Jun Choi. 2023. "In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing" Journal of Functional Biomaterials 14, no. 8: 403. https://doi.org/10.3390/jfb14080403
APA StyleAli, M., Kwak, S. H., Byeon, J. Y., & Choi, H. J. (2023). In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing. Journal of Functional Biomaterials, 14(8), 403. https://doi.org/10.3390/jfb14080403