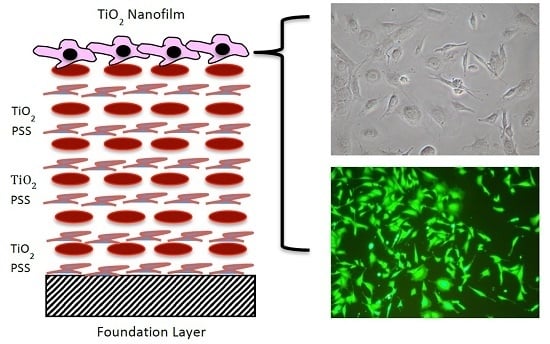
Fibrochondrocyte Growth and Functionality on TiO2 Nanothin Films
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity Assays
2.2. Proliferation Assays
2.2.1. MTT Proliferation Assay
2.2.2. DNA Quantitation Assay
2.3. Protein Assays
2.3.1. Bradford Assay
2.3.2. ELISA
2.4. Statistical Significance
3. Discussion
4. Materials and Methods
4.1. Fibrochondrocyte Isolation and Maintenance
4.2. TiO2 LBL Nanothin Film Formation
4.3. Fibrochondrocyte Seeding on TiO2 Nanosubstrates and Control Substrates
4.4. Live Dead Cytotoxicity Assay
4.5. Proliferation Assays
4.5.1. MTT Proliferation Assay
4.5.2. DNA Quantitation Assay
4.6. Protein Assays
4.6.1. Bradford Assay
4.6.2. ELISA
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kalpakci, K.N.; Willard, V.P.; Athanasiou, K.A. An interspecies comparison of the temporomandibular joint disc. J. Dent. Res. 2011, 90, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.K.; Fiandaca, D.J.; Scapino, R.P. Morphologic, microscopic and immunohistochemical investigations into the function of the primate TMJ disc. J. Orofac. Pain 1994, 8, 136–154. [Google Scholar] [PubMed]
- Milam, S.B. Pathophysiology and Epidemiology of TMJ. J. Musculoskelet. Neuronal Interact. 2003, 3, 382–390. [Google Scholar] [PubMed]
- Mills, D.K.; Daniel, J.C.; Scapino, R.P. Histological features and in vitro proteoglycan synthesis in the rabbit craniomandibular joint disc. Arch Oral Biol. 1988, 33, 195–202. [Google Scholar] [CrossRef]
- Berkovitz, B.K.; Robertshaw, H. Ultrastructural quantification of collagen in the articular disc of the temporomandibular joint of the rabbit. Arch Oral Biol. 1993, 38, 91–95. [Google Scholar] [CrossRef]
- Scapino, R.P.; Canham, P.; Finlay, H.M.; Mills, D.K. The behavior of collagen fibers in stress relaxation and stress distribution in the jaw joint disc of rabbits. Arch Oral Biol. 1996, 41, 1039–1052. [Google Scholar] [CrossRef]
- Taguchi, N.; Nakata, S.; Oka, T. Three-dimensional observation of the temporomandibular joint disk in the rhesus monkey. J. Oral Surg. 1980, 38, 11–15. [Google Scholar] [PubMed]
- Milam, S.B.; Klebe, R.J.; Triplett, R.G.; Herbert, D. Characterization of the extracellular matrix of the primate temporomandibular joint. J. Oral Maxillofac. Surg. 1991, 49, 381–391. [Google Scholar] [CrossRef]
- Fujita, S.; Hoshino, K. Histochemical and immunohistochemical studies on the articular disk of the temporomandibular joint in rats. Acta Anat (Basel) 1989, 134, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Mathema, A.; Mills, D.K. Effects of mechanical shear on anisotropic tissue engineered construct as potential TMJ repair material. FASEB J. 2012, 26, 729. [Google Scholar]
- Allen, K.D.; Athanasiou, K.K. Effect of Passage and Topography on Gene Expression on Temporomandibular Joint Disc Cells. Tissue Eng. 2007, 13, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.K.; Daniel, J.C.; Herzog, S.; Scapino, R.P. An animal model for studying mechanisms in human temporomandibular joint disc derangement. J Oral Maxillofac. Surg. 1994, 52, 1279–1292. [Google Scholar] [CrossRef]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint, etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Ingawaleal, S.; Goswami, T. Temporomandibular joint, disorders, treatments, and biomechanics. Ann. Biomed. Eng. 2009, 37, 976–996. [Google Scholar] [CrossRef] [PubMed]
- Baskan, S.; Zengingul, A. Temporomandibular joint: Disorders and approaches. Biotech. Biotechnol. 2006, 20, 151–155. [Google Scholar] [CrossRef]
- Baird, D.N.; Rea, W.N. The temporomandibular joint implant controversy: A review of autogeneous alloplastic materials and their complications. J. Nutr. Environ. Med. 1998, 8, 289–300. [Google Scholar]
- Willard, V.P.; Zhang, L.; Athanasiou, K.A. Tissue engineering of the temporomandibular joint. In Comprehensive Biomaterials, 1st ed.; Ducheyne, D., Healy, K., Hutmacher, D., Kirkpatrick, J., Eds.; Elsevier: London, UK, 2010; pp. 222–234. [Google Scholar]
- Detamore, M.S.; Athanasiou, K.A. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003, 9, 1065–1087. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.K.; Murphy, M.K.; Masters, T.E.; Hu, J.C.; Athanasiou, K.A. Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell Trans. 2014, 24, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Athanasiou, K.A. Scaffold and growth factor selection in temporomandibular joint disc engineering. J. Dent. Res. 2008, 87, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Webster, T. Nanostructured surface roughness for the control of protein and cell functions. In Handbook of Nanostructured Biomaterials and Their Applications in Nanobiotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2005; Volume 1. [Google Scholar]
- Kommireddy, D.S.; Patel, A.A.; Shutava, G.; Mills, D.K.; Lvov, Y. Layer-by-layer assembly of TiO2 nanoparticles for stable hydrophilic biocompatible coatings. J. Nanosci. Nanotech. 2005, 5, 1081–1087. [Google Scholar] [CrossRef]
- Ben-Nissan, B. Biomedical Thin Films: Mechanical Properties, 1st ed.; Ducheyne, D., Healy, K., Hutmacher, D., Kirkpatrick, J., Eds.; Elsevier: London, UK, 2010; pp. 63–82. [Google Scholar]
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.N.; Jones, F.H.; Olsen, I. Effects of calcium ion implantation on human bone cell interaction with titanium. Biomaterials 2005, 26, 4717–4727. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Trentin, D.; Textor, M.; Tosatti, S.G. Biomedical interfaces, titanium surface technology for implants and cell carriers. Nanomed 2006, 1, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Heimke, G.; Wittal, D.L. Osteointegrated Dental Implants. In Orthopedic, Dental and Bone Graft Applications; Wise, D.L., Trantolo, D.J., Lewandrowski, K., Gresser, J.D., Cattaneo, M.V., Yaszemski, M.J., Eds.; Humana Press: Totowa, NJ, USA, 2000; Volume 1, pp. 67–92. [Google Scholar]
- Torrecillas, R.; Moya, J.S.; Diaz, L.A.; Bartolome, J.F.; Fernandez, A.; Lopez-Esteban, S. Nanotechnology in joint replacement. Nanomed. Nanobiotech. 2009, 1, 540–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdllayev, E.; Lvov, Y. Functional polymer clay nanotube composites with sustained release of chemical agents. Prog. Poly. Sci. 2013, 38, 1690–1719. [Google Scholar]
- Leguen, E.; Chassepot, A.; Decher, G.; Schaaf, P.; Voegel, J.; Jessel, N. Bioactive coatings based on polyelectrolyte multilayer architectures functionalized by embedded proteins, peptides or drugs. Biomol. Eng. 2007, 24, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kommireddy, D.S.; Lvov, Y.; Mills, D.K. Nanoparticle thin films, surface modification for cell attachment and growth. Biomed. Nanotech. 2005, 1, 286–290. [Google Scholar] [CrossRef]
- Jessel, N.; Atalar, F.; Lavalle, J.; Mutterer, G.; Decher, G.; Schaaf, J.; Voegel, J.; Ogier, J. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv. Mat. 2003, 15, 692–695. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, J.; Shen, J. Osteoblast growth promotion by protein electrostatic self assembly on biodegradable poly(lactide). J. Biomat. Sci. 2005, 16, 761–774. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials 2013, 34, 5737–5746. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.M. Mesenchymal stem cell behavior and osteogenic differentiation on layer-by-layer assembled nanoparticle thin films. Master Thesis, Louisiana Tech University, Ruston, LA, USA, 2006. [Google Scholar]
- Kommireddy, D.S.; Sriram, S.M.; Lvov, Y.; Mills, D.K. Stem cell attachment to layer-by-layer assembled TiO2 nanoparticle thin films. Biomaterials 2006, 27, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Chenna, S.G. The Influence of Nanoparticle Thin Films on the Behavior of Osteoblast Cells in vitro. Master Thesis, Louisiana Tech University, Ruston, LA, USA, 2006. [Google Scholar]
- Murphy, M.K.; Macbarb, R.F.; Wong, M.E.; Athanasiou, K. A Temporomandibular joint disorders: A review of etiology, clinical management, and tissue engineering strategies. Int. J. Oral Maxillofac. Implants 2013, 28, e393–e414. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.E.; Wong, M.E.; Athanasiou, K.A. Clinically relevant cell sources for TMJ disc engineering. J. Dent Res. 2008, 87, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Grande, D.; Haug, R.H. Development of an in vitro temporomandibular joint cartilage analog. J. Oral Maxillofac. Surg. 1991, 49, 854–856. [Google Scholar] [CrossRef]
- Puelacher, W.C.; Wisser, J.; Vacanti, C.A.; Ferraro, N.F.; Jaramillo, D.; Vacanti, J.P. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J. Oral Maxillofac. Surg. 1994, 52, 1172–1177. [Google Scholar] [CrossRef]
- Springer, I.N.; Fleiner, B.; Jepsen, S.; Acil, Y. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials 2001, 22, 2569–2577. [Google Scholar] [CrossRef]
- Almarza, A.J.; Athanasiou, K.A. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004, 10, 1787–1095. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Athanasiou, K.A. Passaged goat costal chondrocytes provide a feasible cell source for temporomandibular joint tissue engineering. Ann. Biomed. Eng. 2008, 36, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Athanasiou, K.A. A comparison of primary and passaged chondrocytes for use in engineering the temporomandibular joint. Arch Oral Biol. 2009, 54, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.E.; Athanasiou, K.A. Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell Tissue Res. 2008, 333, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Willard, V.P. Characterization of the Temporomandibular Joint Disc and Fibrocartilage Engineering using Human Embryonic Stem Cells. Ph.D. Thesis, Rice University, Houston, TX, USA, 2012. [Google Scholar]
- Zhang, S.; Yap, A.U.; Toh, W.S. Stem cells for temporomandibular joint repair. Stem Cell Rev. 2015, 11, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Buzarovska, A. PLA nanocomposites with functionalized TiO2 nanoparticles. Poly.-Plast. Technol. Eng. 2013, 52, 280–286. [Google Scholar] [CrossRef]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent, preliminary physico-chemical studies for skin substitute. J. Poly. Res. 2014, 21, 410–415. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Blaker, J.J.; Maquet, M.; Chung, W.; Jerome, R.; Nazhat, S.N. Poly(DL-lactide) (PDLLA) foams with TiO2 nanoparticles and PDLLA/TiO2-bioglass® foam composites for tissue engineering scaffolds. J. Mat. Sci. 2006, 41, 3999–4008. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Jell, A.G.M.; Boccaccini, A.R. Titanium dioxide (TiO2) nanoparticles filled poly(D,L lactid acid) (PDLLA) matrix composites for bone tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 1287–1298. [Google Scholar] [CrossRef] [PubMed]









© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronald, S.; Mills, D.K. Fibrochondrocyte Growth and Functionality on TiO2 Nanothin Films. J. Funct. Biomater. 2016, 7, 15. https://doi.org/10.3390/jfb7020015
Ronald S, Mills DK. Fibrochondrocyte Growth and Functionality on TiO2 Nanothin Films. Journal of Functional Biomaterials. 2016; 7(2):15. https://doi.org/10.3390/jfb7020015
Chicago/Turabian StyleRonald, Sharon, and David K. Mills. 2016. "Fibrochondrocyte Growth and Functionality on TiO2 Nanothin Films" Journal of Functional Biomaterials 7, no. 2: 15. https://doi.org/10.3390/jfb7020015
APA StyleRonald, S., & Mills, D. K. (2016). Fibrochondrocyte Growth and Functionality on TiO2 Nanothin Films. Journal of Functional Biomaterials, 7(2), 15. https://doi.org/10.3390/jfb7020015