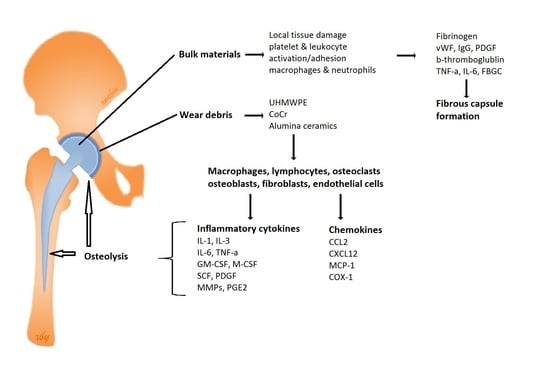
Immunological Responses to Total Hip Arthroplasty
Abstract
:1. Introduction
2. Biological Responses to Bulk Implant Materials
3. Biological Responses to Wear Particles
3.1. Ultra-High Molecular Weight Polyethylene Wear Particles
3.2. Metal Wear Particles
3.3. Alumina Ceramics Wear Particles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kavanagh, B.F.; Dewitz, M.A.; Ilstrup, D.M.; Stauffer, R.N.; Coventry, M.B. Charnley total hip arthroplasty with cement. Fifteen-year results. J. Bone Jt. Surg. 1989, 71, 1496–1503. [Google Scholar] [CrossRef]
- Kandala, N.B.; Connock, M.; Pulikottil-Jacob, R.; Sutcliffe, P.; Crowther, M.J.; Grove, A.; Mistry, H.; Clarke, A. Setting benchmark revision rates for total hip replacement: Analysis of registry evidence. BMJ 2015, 350, h756. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Herberts, P.; Malchau, H. Long-term registration has improved the quality of hip replacement—A review of the Swedish THR Register comparing 160,000 cases. Acta Orthop. Scand. 2000, 71, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.D.; Seyler, T.M.; Bennett, D.; Delanois, R.E.; Saleh, K.J.; Thongtrangan, I.; Kuskowski, M.; Cheng, E.Y.; Sharkey, P.F.; Parvizi, J.; et al. Total hip arthroplasties: What are the reasons for revision? Int. Orthop. 2008, 32, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Ras Sorensen, S.L.; Jorgensen, H.L.; Sporing, S.L.; Lauritzen, J.B. Revision rates for metal-on-metal hip resurfacing and metal-on-metal total hip arthroplasty—A systematic review. Hip Int. 2016, 26, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Raska, M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.J.; Kurtz, S.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J. Bone Jt. Surg. 2009, 91A, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Goodman, S.B. Current state and future of joint replacements in the hip and knee. Expert Rev. Med. Devices 2008, 5, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Bordini, B.; Stea, S.; De Clerico, M.; Strazzari, S.; Sasdelli, A.; Toni, A. Factors affecting aseptic loosening of 4750 total hip arthroplasties: Multivariate survival analysis. BMC Musculoskelet. Disord. 2007, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Dotinga, R. Number of Hip Replacements Has Skyrocketed: Report; WebMD, LLC: Atlanta, GA, USA, 2015. [Google Scholar]
- Zhang, W.; Ouyang, H.W.; Dass, C.R.; Xu, J.K. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [PubMed]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediat. Inflamm. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cobelli, N.; Scharf, B.; Crisi, G.M.; Hardin, J.; Santambrogio, L. Mediators of the inflammatory response to joint replacement devices. Nat. Rev. Rheumatol. 2011, 7, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Chun, A.L. Implant materials: Reducing wear debris. Nat. Nanotechnol. 2016. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, P.R.T.; Schemitsch, E.H. The basic science of peri-implant bone healing. Indian J. Orthop. 2011, 45, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraja, S.; Lundstrom, I.; Nygren, H.; Tengvall, P. Platelet binding and protein adsorption to titanium and gold after short time exposure to heparinized plasma and whole blood. Biomaterials 1996, 17, 2225–2232. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological responses to materials. Ann. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E.; Delmaschio, A. Platelet-dependent modulation of neutrophil function. Pharmacol. Res. 1992, 26, 269–272. [Google Scholar] [CrossRef]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- DeFife, K.M.; Jenney, C.R.; McNally, A.K.; Colton, E.; Anderson, J.M. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J. Immunol. 1997, 158, 3385–3390. [Google Scholar] [PubMed]
- Kao, W.Y.J.; Mcnally, A.K.; Hiltner, A.; Anderson, J.M. Role for interleukin-4 in foreign-body giant-cell formation on a poly(etherurethane urea) in vivo. J. Biomed. Mater. Res. 1995, 29, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Grainger, D.W. All charged up about implanted biomaterials. Nat. Biotechnol. 2013, 31, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Brach Del Prever, E.M.; Bistolfi, A.; Bracco, P.; Costa, L. Uhmwpe for arthroplasty: Past or future? J. Orthop. Traumatol. 2009, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-S.; Greer, K.; Hanes, M.; McNulty, D. Effects of resin and dose on wear and mechanical properties of cross-linked thermally stabilized uhmwpe. In Proceedings of the 7th World Biomaterials Congress-2004, Sydney, Australia, 17–21 May 2004. [Google Scholar]
- Jiang, Y.P.; Jia, T.H.; Wooley, P.H.; Yang, S.Y. Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris. Acta Orthop. Belg. 2013, 79, 1–9. [Google Scholar] [PubMed]
- Zaveri, T.D.; Dolgova, N.V.; Lewis, J.S.; Hamaker, K.; Clare-Salzler, M.J.; Keselowsky, B.G. Macrophage integrins modulate response to ultra-high molecular weight polyethylene particles and direct particle-induced osteolysis. Biomaterials 2017, 115, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Margevicius, K.J.; Bauer, T.W.; Mcmahon, J.T.; Brown, S.A.; Merritt, K. Isolation and characterization of debris in membranes around total joint prostheses. J. Bone Jt. Surg. 1994, 76A, 1664–1675. [Google Scholar] [CrossRef]
- Suñer, S.; Tipper, J.L.; Emami, N. Biological effects of wear particles generated in total joint replacements: Trends and future prospects. Tribol. Mater. Surf. Interfaces 2012, 6, 39–52. [Google Scholar] [CrossRef]
- Rao, A.J.; Gibon, E.; Ma, T.; Yao, Z.Y.; Smith, R.L.; Goodman, S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012, 8, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.; Fisher, J. Biological reactions to wear debris in total joint replacement. Proc. Inst. Mech. Eng. Part H 2000, 214, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by il-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Rosenberg, E.; de Papp, A.E.; Duong le, T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Investig. 2012, 42, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Ma, T.; Ren, P.G.; Fritton, K.; Biswal, S.; Yao, Z.; Smith, L.; Goodman, S.B. Selective inhibition of the mcp-1-ccr2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of uhmwpe particles. J. Orthop. Res. 2012, 30, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [PubMed]
- Koreny, T.; Tunyogi-Csapo, M.; Gal, I.; Vermes, C.; Jacobs, J.J.; Glant, T.T. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum. 2006, 54, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Raynal, N.; Andersen, T.L.; Slatter, D.A.; Bihan, D.; Pugh, N.; Cella, M.; Kim, T.; Rho, J.; Negishi-Koga, T.; et al. Oscar is a collagen receptor that costimulates osteoclastogenesis in dap12-deficient humans and mice. J. Clin. Investig. 2011, 121, 3505–3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulus, A.C.; Haßelt, S.; Jansson, V.; Giurea, A.; Neuhaus, H.; Grupp, T.M.; Utzschneider, S. Histopathological analysis of peek wear particle effects on the synovial tissue of patients. BioMed Res. Int. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Doorn, P.F.; Campbell, P.A.; Worrall, J.; Benya, P.D.; McKellop, H.A.; Amstutz, H.C. Metal wear particle characterization from metal on metal total hip replacements: Transmission electron microscopy study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. 1998, 42, 103–111. [Google Scholar] [CrossRef]
- Galvin, A.L.; Tipper, I.L.; Jennings, L.M.; Stone, M.H.; Jin, Z.M.; Ingham, E.; Fisher, J. Wear and biological activity of highly crosslinked polyethylene in the hip under low serum protein concentrations. Proc. Inst. Mech. Eng. Part H 2007, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, I.D.; Gheduzzi, S.; Vail, T.P. Clinical experience with metal-on-metal total joint replacements: Indications and results. Proc. Inst. Mech. Eng. Part H 2006, 220, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Jt. Surg. 2000, 82, 457–476. [Google Scholar] [CrossRef]
- Christian, W.V.; Oliver, L.D.; Paustenbach, D.J.; Kreider, M.L.; Finley, B.L. Toxicology-based cancer causation analysis of cocr-containing hip implants: A quantitative assessment of genotoxicity and tumorigenicity studies. J. Appl. Toxicol. 2014, 34, 939–967. [Google Scholar] [CrossRef] [PubMed]
- Rakow, A.; Schoon, J.; Dienelt, A.; John, T.; Textor, M.; Duda, G.; Perka, C.; Schulze, F.; Ode, A. Influence of particulate and dissociated metal-on-metal hip endoprosthesis wear on mesenchymal stromal cells in vivo and in vitro. Biomaterials 2016, 98, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wei, W.; Yue, Z.; Lv, P.; Wang, L.; Ma, G.; Su, Z. Particle size affects the cellular response in macrophages. Eur. J. Pharm. Sci. 2010, 41, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Messer, R.L.; Bishop, S.; Lucas, L.C. Effects of metallic ion toxicity on human gingival fibroblasts morphology. Biomaterials 1999, 20, 1647–1657. [Google Scholar] [CrossRef]
- Wang, N.; Kunz, J.L.; Ivey, C.D.; Ingersoll, C.G.; Barnhart, M.C.; Glidewell, E.A. Toxicity of chromium (vi) to two mussels and an amphipod in water-only exposures with or without a co-stressor of elevated temperature, zinc, or nitrate. Arch. Environ. Contam. Toxicol. 2017, 72, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Dalal, N.S. Generation of hydroxyl radical by chromate in biologically relevant systems: Role of cr(v) complexes versus tetraperoxochromate(v). Environ. Health Perspect. 1994, 102, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Aaseth, J. Uptake of chromate in human red blood cells and isolated rat liver cells: The role of the anion carrier. Analyst 1995, 120, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Grammatopoulos, G.; Adshead, S.; Tsialogiannis, E.; Tsiridis, E. Molecular and immune toxicity of cocr nanoparticles in mom hip arthroplasty. Trends Mol. Med. 2012, 18, 145–155. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Surgical Implants and Other Foreign Bodies; The International Agency for Research on Cancer (IARC): Lyon, France, 1999; Volume 74, pp. 1–409. [Google Scholar]
- Doherty, A.T.; Howell, R.T.; Ellis, L.A.; Bisbinas, I.; Learmonth, I.D.; Newson, R.; Case, C.P. Increased chromosome translocations and aneuploidy in peripheral blood lymphocytes of patients having revision arthroplasty of the hip. J. Bone Jt. Surg. 2001, 83, 1075–1081. [Google Scholar] [CrossRef]
- Davies, A.P.; Sood, A.; Lewis, A.C.; Newson, R.; Learmonth, D.; Case, C.P. Metal-specific differences in levels of DNA damage caused by synovial fluid recovered at revision arthroplasty. J. Bone Jt. Surg. 2005, 87B, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, E.; Ladon, D.; Whittingharn-Jones, P.; Carrington, R.; Briggs, T.W. Chromosomal aberrations in the peripheral blood of patients with metal-on-metal hip bearings. J. Bone Jt. Surg. 2008, 90A, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Visuri, T.I.; Pukkala, E.; Pulkkinen, P.; Paavolainen, P. Cancer incidence and causes of death among total hip replacement patients: A review based on nordic cohorts with a special emphasis on metal-on-metal bearings. Proc. Inst. Mech. Eng. Part H 2006, 220, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Beaule, P.E.; Campbell, P.; Mirra, J.; Hooper, J.C.; Schmalzried, T.P. Osteolysis in a cementless, second generation metal-on-metal hip replacement. Clin. Orthop. Relat. Res. 2001, 386, 159–165. [Google Scholar] [CrossRef]
- Huber, M.; Reinisch, G.; Zenz, P.; Zweymuller, K.; Lintner, F. Postmortem study of femoral osteolysis associated with metal-on-metal articulation in total hip replacement an analysis of nine cases. J. Bone Jt. Surg. 2010, 92A, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Fehring, K.A.; Fehring, T.K. Modes of failure in metal-on-metal total hip arthroplasty. Orthop. Clin. N. Am. 2015, 46, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Pandit, H.; Vlychou, M.; Whitwell, D.; Crook, D.; Luqmani, R.; Ostlere, S.; Murray, D.W.; Athanasou, N.A. Necrotic granulomatous pseudotumours in bilateral resurfacing hip arthoplasties: Evidence for a type iv immune response. Virchows Arch. 2008, 453, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Koper, M.C.; Mathijssen, N.M.C.; Vehmeijer, S.B.W. A 5-year survival analysis of 160 biomet magnum m2 metal-on-metal total hip prostheses. Hip Int. 2016, 26, 50. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Ebramzadeh, E.; Nelson, S.; Takamura, K.; De Smet, K.; Amstutz, H.C. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin. Orthop. Relat. Res. 2010, 468, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Mikecz, K.; Vermes, C.; Skipor, A.; Jacobs, J.J. Orthopaedic implant related metal toxicity in terms of human lymphocyte reactivity to metal-protein complexes produced from cobalt-base and titanium-base implant alloy degradation. Mol. Cell. Biochem. 2001, 222, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Anderson, S.; Caicedo, M.; Skipor, A.; Campbell, P.; Jacobs, J.J. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J. Arthroplast. 2004, 19, 88–93. [Google Scholar] [CrossRef]
- Catelas, I.; Petit, A.; Zukor, D.J.; Antoniou, J.; Huk, O.L. Tnf-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials 2003, 24, 383–391. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Hallab, N.J. Loosening and osteolysis associated with metal-on-metal bearings: A local effect of metal hypersensitivity? J. Bone Jt. Surg. 2006, 88A, 1171–1172. [Google Scholar] [CrossRef]
- Sáenz, A.; Rivera-Muño, E.; Brostow, W.; Castaño, M.V. Ceramic biomaterials: An introductory overview. J. Mater. Educ. 1999, 21, 297–306. [Google Scholar]
- Sedel, L. Evolution of alumina-on-alumina implants-a review. Clin. Orthop. Relat. Res. 2000, 379, 48–54. [Google Scholar] [CrossRef]
- Hatton, A.; Nevelos, J.E.; Nevelos, A.A.; Banks, R.E.; Fisher, J.; Ingham, E. Alumina-alumina artificial hip joints. Part I: A histological analysis and characterisation of wear debris by laser capture microdissection of tissues retrieved at revision. Biomaterials 2002, 23, 3429–3440. [Google Scholar] [CrossRef]
- Tipper, J.L.; Hatton, A.; Nevelos, J.E.; Ingham, E.; Doyle, C.; Streicher, R.; Nevelos, A.B.; Fisher, J. Alumina-alumina artificial hip joints. Part II: Characterisation of the wear debris from in vitro hip joint simulations. Biomaterials 2002, 23, 3441–3448. [Google Scholar] [CrossRef]
- Jarrett, C.A.; Ranawat, A.S.; Bruzzone, M.; Blum, Y.C.; Rodriguez, J.A.; Ranawat, C.S. The squeaking hip: A phenomenon of ceramic-on-ceramic total hip arthroplasty. J. Bone Jt. Surg. 2009, 91A, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I.; Petit, A.; Zukor, D.J.; Marchand, R.; Yahia, L.; Huk, O.L. Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials 1999, 20, 625–630. [Google Scholar] [CrossRef]
- Brown, C.; Williams, S.; Tipper, J.L.; Fisher, J.; Ingham, E. Characterisation of wear particles produced by metal on metal and ceramic on metal hip prostheses under standard and microseparation simulation. J. Mater. Sci. 2007, 18, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Warashina, H.; Sakano, S.; Kitamura, S.; Yamauchi, K.I.; Yamaguchi, J.; Ishiguro, N.; Hasegawa, Y. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials 2003, 24, 3655–3661. [Google Scholar] [CrossRef]
- Christel, P.S. Biocompatibility of surgical-grade dense polycrystalline alumina. Clin. Orthop. Relat. Res. 1992, 10–18. [Google Scholar] [CrossRef]
- Germain, M.A.; Hatton, A.; Williams, S.; Matthews, J.B.; Stone, M.H.; Fisher, J.; Ingham, E. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials 2003, 24, 469–479. [Google Scholar] [CrossRef]
- Hannouche, D.; Hamadouche, M.; Nizard, R.; Bizot, P.; Meunier, A.; Sedel, L. Ceramics in total hip replacement. Clin. Orthop. Relat. Res. 2005, 430, 62–71. [Google Scholar] [CrossRef]
- Tsaousi, A.; Jones, E.; Case, C.P. The in vitro genotoxicity of orthopaedic ceramic (Al2O3) and metal (cocr alloy) particles. Mutat. Res. 2010, 697, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Catelas, I.; Antoniou, J.; Zukor, D.J.; Huk, O.L. Differential apoptotic response of j774 macrophages to alumina and ultra-high-molecular-weight polyethylene particles. J. Orthop. Res. 2002, 20, 9–15. [Google Scholar] [CrossRef]
- Hatton, A.; Nevelos, J.E.; Matthews, J.B.; Fisher, J.; Ingham, E. Effects of clinically relevant alumina ceramic wear particles on tnf-alpha production by human peripheral blood mononuclear phagocytes. Biomaterials 2003, 24, 1193–1204. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, K.; Jiang, L.-H.; Foster, R.; Yang, X.B. Immunological Responses to Total Hip Arthroplasty. J. Funct. Biomater. 2017, 8, 33. https://doi.org/10.3390/jfb8030033
Man K, Jiang L-H, Foster R, Yang XB. Immunological Responses to Total Hip Arthroplasty. Journal of Functional Biomaterials. 2017; 8(3):33. https://doi.org/10.3390/jfb8030033
Chicago/Turabian StyleMan, Kenny, Lin-Hua Jiang, Richard Foster, and Xuebin B Yang. 2017. "Immunological Responses to Total Hip Arthroplasty" Journal of Functional Biomaterials 8, no. 3: 33. https://doi.org/10.3390/jfb8030033
APA StyleMan, K., Jiang, L. -H., Foster, R., & Yang, X. B. (2017). Immunological Responses to Total Hip Arthroplasty. Journal of Functional Biomaterials, 8(3), 33. https://doi.org/10.3390/jfb8030033