Electron Beam Immobilization of Novel Antimicrobial, Short Peptide Motifs Leads to Membrane Surfaces with Promising Antibacterial Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of New IL-KKA Variants
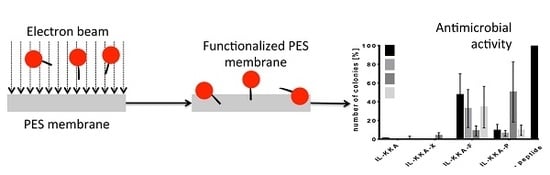
2.2. Electron Beam Modification of PES Membranes
2.3. Antimicrobial Activity
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Solid-Phase Peptide Synthesis
3.3. Immobilization of Different Peptides on PES Membranes
3.4. Membrane Characterization
3.5. Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.D. Modality of bacterial growth presents unique targets: How do we treat biofilm-mediated infections? Curr. Opin. Microbiol. 2010, 13, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Harro, J.M.; Peters, B.M.; O’May, G.A.; Archer, N.; Kerns, P.; Prabhakara, R.; Shirtliff, M.E. Vaccine development in staphylococcus aureus: Taking the biofilm phenotype into consideration. FEMS Immunol. Med. Microbiol. 2010, 59, 306–323. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C. Biofilms: Recent developments on an old battle. Recent Patents Biotechnol. 2007, 1, 49–57. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo, A.C.; Camargo, E.R.; Barbosa, D.B. The growing importance of materials that prevent microbial adhesion: Antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Harbers, G.M.; Emoto, K.; Greef, C.; Metzger, S.W.; Woodward, H.N.; Mascali, J.J.; Grainger, D.W.; Lochhead, M.J. Functionalized poly(ethylene glycol)-based bioassay surface chemistry that facilitates bio-immobilization and inhibits nonspecific protein, bacterial, and mammalian cell adhesion. Chem. Mater. 2007, 19, 4405–4414. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.G.; Ostuni, E.; Liang, M.N.; Meluleni, G.; Kim, E.; Yan, L.; Pier, G.; Warren, H.S.; Whitesides, G.M. Polymeric thin films that resist the adsorption of proteins and the adhesion of bacteria. Langmuir 2001, 17, 1225–1233. [Google Scholar] [CrossRef]
- Goncalves, I.C.; Martins, M.C.L.; Barbosa, M.A.; Naeemi, E.; Ratner, B.D. Selective protein adsorption modulates platelet adhesion and activation to oligo(ethylene glycol)-terminated self-assembled monolayers with c18 ligands. J. Biomed. Mater. Res. A 2009, 89, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Nishimura, N.; Kamata, H.; Furue, K.; Ono, Y.; Hosomi, M.; Terada, A. Antibacterial and anti-biofilm efficacy of fluoropolymer coating by a 2,3,5,6-tetrafluoro-p-phenylenedimethanol structure. Colloids Surf. B Biointerfaces 2017, 151, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.W.; Papadakis, S.E.; Yam, K.L. Evaluation of a polymer coating containing triclosan as the antimicrobial layer for packaging materials. Int. J. Food Sci. Technol. 2003, 38, 165–169. [Google Scholar] [CrossRef]
- Ravikumar, T.; Murata, H.; Koepsel, R.R.; Russell, A.J. Surface-active antifungal polyquaternary amine. Biomacromolecules 2006, 7, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Gallarato, L.A.; Mulko, L.E.; Dardanelli, M.S.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Synergistic effect of polyaniline coverage and surface microstructure on the inhibition of Pseudomonas aeruginosa biofilm formation. Colloids Surf. B Biointerfaces 2017, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aumsuwan, N.; Danyus, R.C.; Heinhorst, S.; Urban, M.W. Attachment of ampicilin to explanded poly(tetrafluoethylene) (eptfe): Surface reactions leading to inhibition of microbial growth. Biomacromolecules 2008, 9, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Cevher, E.; Orhan, Z.; Mulazimoglu, L.; Sensoy, D.; Alper, M.; Yildiz, A.; Ozsoy, Y. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with prepared microspheres. Int. J. Pharm. 2006, 317, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Aumsuwan, N.; Heinhorst, S.; Urban, M.W. Antibacterial surfaces on expanded polytetrafluoroethylene; penicillin attachment. Biomacromolecules 2007, 8, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Bayramov, D.F.; Neff, J.A. Beyond conventional antibiotics—New directions for combination products to combat biofilm. Adv. Drug Deliv. Rev. 2017, 112, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti-Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C.; Hristova, K. Antimicrobial peptides: Successes, challenges and unanswered questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Maria-Neto, S.; de Almeida, K.C.; Macedo, M.L.R.; Franco, O.L. Understanding bacterial resistance to antimicrobial peptides: From the surface to deep inside. Biochim. Biophys. Acta Biomembr. 2015, 1848, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Neundorf, I. Design and application of antimicrobial peptide conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Lushnikova, T.; Golla, R.M.; Wang, X.; Wang, G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017, 49, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Marchand-Brynaert, J. Polymer Membranes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Fernandez-Fernandez, M.; Sanroman, M.A.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Bhattacharyya, D.; Daunert, S.; Bachas, L. Catalytic biofunctional membranes containing site-specifically immobilized enzyme arrays: A review. J. Membr. Sci. 2001, 181, 29–37. [Google Scholar] [CrossRef]
- Hicke, H.G.; Ulbricht, M.; Becker, M.; Radosta, S.; Heyer, A.G. Novel enzyme-membrane reactor for polysaccharide synthesis. J. Membr. Sci. 1999, 161, 239–245. [Google Scholar] [CrossRef]
- Tischer, W.; Wedekind, F. Immobilized enzymes: Methods and applications. Top. Curr. Chem. 1999, 200, 95–126. [Google Scholar] [CrossRef]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods employed for control of fouling in mf and uf membranes: A comprehensive review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric membranes: Surface modification for minimizing (bio)colloidal fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Marquardt, B.; Kaczmarek, S.; Schubert, R.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of poly(ethersulfone) membranes. Macromol. Rapid Commun. 2010, 31, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Marquardt, B.; Went, M.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of polymer membranes. Water Sci. Technol. 2012, 65, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Maitz, M.F.; Zimmermann, R.; Marquardt, B.; Fischer, M.; Werner, C.; Went, M.; Thomas, I. Permanent surface modification by electron-beam-induced grafting of hydrophilic polymers to PVDF membranes. RSC Adv. 2013, 3, 22518–22526. [Google Scholar] [CrossRef]
- Starke, S.; Went, M.; Prager, A.; Schulze, A. A novel electron beam-based method for the immobilization of trypsin on poly(ethersulfone) and poly(vinylidene fluoride) membranes. React. Funct. Polym. 2013, 73, 698–702. [Google Scholar] [CrossRef]
- Jahangiri, E.; Reichelt, S.; Thomas, I.; Hausmann, K.; Schlosser, D.; Schulze, A. Electron beam-induced immobilization of laccase on porous supports for waste water treatment applications. Molecules 2014, 19, 11860–11882. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Stoelzer, A.; Striegler, K.; Starke, S.; Prager, A. Biocatalytic self-cleaning polymer membranes. Polymers 2015, 7, 1837–1849. [Google Scholar] [CrossRef]
- Schulze, A.; Breite, D.; Kim, Y.; Schmidt, M.; Thomas, I.; Went, M.; Fischer, K.; Prager, A. Bio-inspired polymer membrane surface cleaning. Polymers 2017, 9, 97. [Google Scholar] [CrossRef]
- Postleb, F.; Stefanik, D.; Seifert, H.; Giernoth, R. BIOnic liquids: Imidazolium-based ionic liquids with antimicrobial activity. Z. Naturforsch. B 2013, 68, 1123–1128. [Google Scholar] [CrossRef]
- Tietze, A.A.; Bordusa, F.; Giernoth, R.; Imhof, D.; Lenzer, T.; Maass, A.; Mrestani-Klaus, C.; Neundorf, I.; Oum, K.; Reith, D.; et al. On the nature of interactions between ionic liquids and small amino-acid-based biomolecules. ChemPhysChem 2013, 14, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Horn, M.; Schmauck, J.P.G.; Brohl, A.; Giernoth, R.; Oelkrug, C.; Schubert, A.; Neundorf, I. Novel imidazolium salt-peptide conjugates and their antimicrobial activity. Bioconjug. Chem. 2014, 25, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.J.; Lee, Y.S.; Song, M.D.; Kim, I.H.; Won, H.S. De novo generation of short antimicrobial peptides with simple amino acid composition. Regul. Pept. 2011, 166, 36–41. [Google Scholar] [CrossRef] [PubMed]




| Name | Sequence | MWcalc [Da] | MWexp [Da] | Net Charge | RT [min] | Purity [%] |
|---|---|---|---|---|---|---|
| IL-KKA | IL4-KK(εK)βA-OH | 1751.7 | 1752.2 | +4 | 18.78 | 98 |
| IL-KKA-X | IL4-KK(εK)βA-Ahx-Ahx-Gly-OH | 2035.1 | 2035.7 | +4 | 15.63 | 98 |
| IL-KKA-F | IL4-KK(εK)βA-Phe-Phe-OH | 2046.1 | 2046.8 | +4 | 15.08 | 95 |
| IL-KKA-P | IL4-KK(εK)βA-Trp-Leu-Leu-Lys-Trp-OH | 2478.7 | 2479.6 | +5 | 13.75 | 97 |
| Elemental Ratio (Relative Atom-%) | CA [°] | ||||
|---|---|---|---|---|---|
| Label | C | N | O | S | |
| PES Membrane (reference) | 69.95 | - | 26.12 | 3.93 | 55.3 |
| Membrane + IL-KKA 50 kGy | 67.81 | 0.69 | 28.17 | 3.33 | 45.2 |
| Membrane + IL-KKA 100 kGy | 69.25 | 0.25 | 26.87 | 3.62 | 53.5 |
| Membrane + IL-KKA 150 kGy | 69.39 | 0.4 | 27.05 | 3.56 | 60.4 |
| Membrane + IL-KKA (NHS/EDC) | 69.61 | 1.02 | 26.61 | 3.78 | 44.8 |
| Membrane + IL-KKA-X 50 kGy | 69.28 | 0.71 | 26.85 | 3.17 | 54.2 |
| Membrane + IL-KKA-X 100 kGy | 69.44 | 0.69 | 26.61 | 3.27 | 56.4 |
| Membrane + IL-KKA-X 150 kGy | 68.79 | 0.17 | 27.47 | 3.56 | 58.7 |
| Membrane + IL-KKA-X (NHS/EDC) | 69.42 | 1.03 | 26.50 | 3.06 | 50.7 |
| Membrane + IL-KKA-F 50 kGy | 68.38 | 0.81 | 27.53 | 3.28 | 48.1 |
| Membrane + IL-KKA-F 100 kGy | 67.84 | 0.59 | 28.20 | 3.37 | 60.2 |
| Membrane + IL-KKA-F 150 kGy | 69.55 | 1.34 | 25.86 | 3.25 | 61.5 |
| Membrane + IL-KKA-F (NHS/EDC) | 68.95 | 0.33 | 27.06 | 3.65 | 46.1 |
| Membrane + IL-KKA-P 50 kGy | 68.81 | 0.72 | 27.12 | 3.35 | 58.0 |
| Membrane + IL-KKA-P 100 kGy | 68.60 | 0.73 | 27.34 | 3.33 | 58.1 |
| Membrane + IL-KKA-P 150 kGy | 70.51 | 1.73 | 24.93 | 2.85 | 68.1 |
| Membrane + IL-KKA-P (NHS/EDC) | 69.15 | 0.73 | 26.79 | 3.33 | 49.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinhardt, A.; Thomas, I.; Schmauck, J.; Giernoth, R.; Schulze, A.; Neundorf, I. Electron Beam Immobilization of Novel Antimicrobial, Short Peptide Motifs Leads to Membrane Surfaces with Promising Antibacterial Properties. J. Funct. Biomater. 2018, 9, 21. https://doi.org/10.3390/jfb9010021
Reinhardt A, Thomas I, Schmauck J, Giernoth R, Schulze A, Neundorf I. Electron Beam Immobilization of Novel Antimicrobial, Short Peptide Motifs Leads to Membrane Surfaces with Promising Antibacterial Properties. Journal of Functional Biomaterials. 2018; 9(1):21. https://doi.org/10.3390/jfb9010021
Chicago/Turabian StyleReinhardt, André, Isabell Thomas, Julie Schmauck, Ralf Giernoth, Agnes Schulze, and Ines Neundorf. 2018. "Electron Beam Immobilization of Novel Antimicrobial, Short Peptide Motifs Leads to Membrane Surfaces with Promising Antibacterial Properties" Journal of Functional Biomaterials 9, no. 1: 21. https://doi.org/10.3390/jfb9010021
APA StyleReinhardt, A., Thomas, I., Schmauck, J., Giernoth, R., Schulze, A., & Neundorf, I. (2018). Electron Beam Immobilization of Novel Antimicrobial, Short Peptide Motifs Leads to Membrane Surfaces with Promising Antibacterial Properties. Journal of Functional Biomaterials, 9(1), 21. https://doi.org/10.3390/jfb9010021