Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
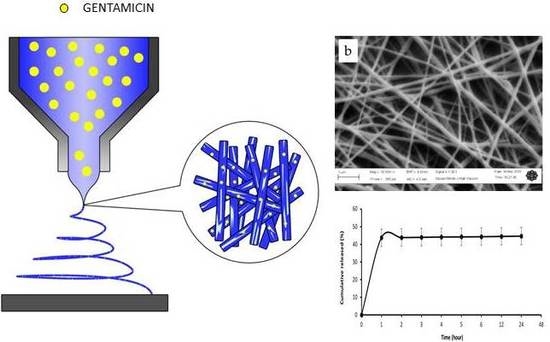
2.2. Preparation of Electrospun Fibres
2.3. Morphological Characterization
2.4. In Vitro Drug Release
2.5. Cell Culture
2.6. In Vitro Cytotoxicity Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis
3.2. In Vitro Drug Release
3.3. In Vitro Cytotoxicity Test
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tenenhaus, M.; Rennekampff, H.O. Current Concepts in Tissue Engineering. Plast. Reconstr. Surg. 2016, 138, 42S–50S. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.A.; Ong, J.F.; Eriksson, E.; Junker, J.P.; Caterson, E.J. Tissue engineering of skin. J. Am. Coll. Surg. 2013, 217, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Gloria, A.; Raucci, M.G.; Ambrosio, L. Hydrogel-based platforms for the regeneration of osteochondral tissue and intervertebral disc. Polymers 2012, 4, 1590–1612. [Google Scholar] [CrossRef]
- Aguirre-Chagala, Y.E.; Altuzar, V.; León-Sarabia, E.; Tinoco-Magaña, J.C.; Yañez-Limón, J.M.; Mendoza-Barrera, C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater. Sci. Eng. C 2017, 76, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Alvarez-Perez, M.; Cirillo, V.; Ambrosio, L. HMSC interaction with PCL and PCL/gelatin platforms: A comparative study on films and electrospun membranes. J. Bioact. Compat. Polym. 2011, 26, 144–160. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Altobelli, R.; Ambrosio, L. Polymer-based platforms by electric field-assisted techniques for tissue engineering and cancer therapy. Expert Rev. Med. Devices 2014, 12, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Basar, A.O.; Castro, S.; Torres-Giner, S.; Lagaron, J.M.; Turkoglu Sasmazel, H. Novel poly(ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater. Sci. Eng. C 2017, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design ECM tissue analogues. Exp. Rev. Med. Dev. 2016, 13, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Jao, W.C.; Yang, M.C.; Lin, C.H.; Hsu, C.C. Fabrication and characterization of electrospun silk fibroin/TiO2 nanofibrous mats for wound dressings. Polym. Adv. Technol. 2012, 23, 1066–1076. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Wan Abdul Khodir, W.K.; Guarino, M.A.; Alvarez-Perez, M.A.; Cafiero, C.; Ambrosio, L. Trapping tetracycline-loaded nanoparticles into polycaprolactone fiber networks for periodontal regeneration therapy. J. Bioact. Compat. Polym. 2013, 28. [Google Scholar] [CrossRef]
- Guadalupe, E.; Ramos, D.; Shelke, N.B.; James, R.; Gibney, C.; Kumbar, S.G. Bioactive polymeric nanofiber matrices for skin regeneration. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen Scaffolds for Tissue Engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of type I collagen and PCL nanofibers using acetic acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Baylan, N.; Bhat, S.; Ditto, M.; Lawrence, J.G.; Lecka-Czernik, B.; Yildirim-Ayan, E. Polycaprolactone nanofiber interspersed collagen type-I scaffold for bone regeneration: A unique injectable osteogenic scaffold. Biomed. Mater. (Bristol) 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.M.; Dang, T.N.T.; Tsai, E.C.; Cao, X. Electrospun biocomposite polycaprolactone/collagen tubes as scaffolds for neural stem cell differentiation. Materials 2010, 3, 3714–3728. [Google Scholar] [CrossRef]
- Venugopal, J.; Prabhakaran, M.P.; Low, S.; Choon, A.T.; Deepika, G.; Giri Dev, V.R.; Ramakrishna, S. Continuous nanostructures for the controlled release of drugs. Curr. Pharm. Des. 2009, 15, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; McColgan-Bannon, K.; Gianone, N.C.; Sefat, F.; Dalgarno, K.; Ferreira, A.M. Biosynthetic PCL-graft-Collagen Bulk Material for Tissue Engineering Applications. Materials 2017, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińsk, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. Engineered human skin fabricated using electrospun collagen–PCL blends: Morphogenesis and mechanical properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K; Tong, Y.W.; Yung, L.Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunah, M. Electro-spinning of pure collagen nanofibres—Just an expensive way to make gelatin. Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Guarino, V.; Alvarez-Perez, M.A.; Marrese, M.; Ambrosio, L. Optimization of fully aligned bioactive electrospun fibers for “in vitro” nerve guidance. J. Mater. Sci. Mater. Med. 2014, 25, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.; Mendes, A.; Bártolo, P.J. PCL scaffolds with collagen bioactivator for applications in Tissue Engineering. Procedia Eng. 2013, 5, 279–284. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly (ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yeo, M.; Ahn, S.; Kang, D.O.; Jang, C.H.; Lee, H.; Park, G.M.; Kim, G.H. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sirc, J.; Kubinova, S.; Hobzova, R.; Stranska, D.; Kozlik, P.; Bosakova, Z.; Michalek, J. Controlled gentamicin release from multi-layered electrospun nanofibrous structures of various thicknesses. Int. J. Nanomed. 2012, 7, 5315–5325. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, M.; Martins, A.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Antibacterial activity of chitosan nanofiber meshes with liposomes immobilized releasing gentamicin. Acta Biomater. 2015, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Lecároz, C.; Campanero, M.A.; Gamazo, C.; Blanco-Prieto, J.M. Determination of gentamycin in different matrices by a new sensitive high-performance liquid chromatography-mass spectrometric method. J. Antimicrob. Chemother. 2006, 58, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Guaccio, A.; Guarino, V.; Perez, M.A.A.; Cirillo, V.; Netti, P.A.; Ambrosio, L. Influence of electrospun fiber mesh size on hMSC oxygen metabolism in 3D collagen matrices: Experimental and theoretical evidences. Biotechnol. Bioeng. 2011, 108, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of Collagen from skin, scale and bone of Carp (Cyprinus carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Guarino, V.; Cruz-Maya, I.; Altobelli, R.; Abdul Khodir, W.K.; Ambrosio, L.; Pèrez, M.A.A.; Flores, A.A. Electrospun Polycaprolactone nanofibres decorated by drug loaded chitosan nano-reservoirs for antibacterial treatments. Nanotechnology 2017, 28, 505103. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Gloria, A.; Raucci, MG.; De Santis, R.; Ambrosio, L. Bio-inspired cell instructive composite platforms for bone regeneration. Int. Mater. Rev. 2012, 57, 256–275. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Biocompatible Nanofiber Matrices for the Engineering of a Dermal Substitute for Skin Regeneration. Tissue Eng. 2005, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Arbabi Bidgoli, S.; Rezayat, M. Electrospun polymeric nanofibers for transdermal drug delivery. Nanomed. J. 2017, 4, 61–70. [Google Scholar] [CrossRef]
- Puppi, D.; Dinucci, D.; Bartoli, C.; Mota, C.; Migone, C.; Dini, F.; Barsotti, G.; Carlucci, F.; Chiellini, F. Development of 3D wet-spun polymeric scaffolds loaded with antimicrobial agents for bone engineering. J. Bioact. Compat. Polym. 2011, 26, 478–492. [Google Scholar] [CrossRef]
- Barrientos, I.J.H.; Paladino, E.; Brozio, S.; Passarelli, M.; Moug, S.; Black, R.A.; Wilson, C.G.; Lamprou, D.A. Fabrication and characterisation of drug-loaded electrospun polymeric nanofibers for controlled release in hernia repair. Int. J. Pharm. 2016, 517, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Venugopal, J.R.; Navaneethan, B.; Laiva, A.L.; Ramakrishna, S. Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater. Lett. 2015, 141, 180–186. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Khodir, W.K.W.; Abdul Razak, A.H.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. https://doi.org/10.3390/jfb9020036
Abdul Khodir WKW, Abdul Razak AH, Ng MH, Guarino V, Susanti D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. Journal of Functional Biomaterials. 2018; 9(2):36. https://doi.org/10.3390/jfb9020036
Chicago/Turabian StyleAbdul Khodir, Wan Khartini Wan, Abdul Hakim Abdul Razak, Min Hwei Ng, Vincenzo Guarino, and Deny Susanti. 2018. "Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration" Journal of Functional Biomaterials 9, no. 2: 36. https://doi.org/10.3390/jfb9020036
APA StyleAbdul Khodir, W. K. W., Abdul Razak, A. H., Ng, M. H., Guarino, V., & Susanti, D. (2018). Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. Journal of Functional Biomaterials, 9(2), 36. https://doi.org/10.3390/jfb9020036