Biomimetic vs. Direct Approach to Deposit Hydroxyapatite on the Surface of Low Melting Point Polymers for Tissue Engineering
Abstract
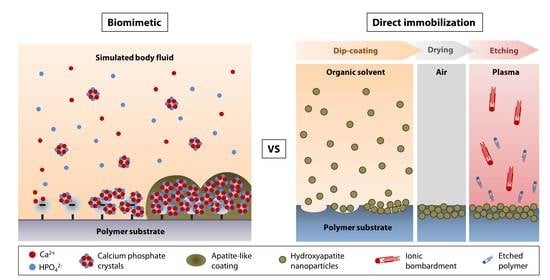
:1. Introduction
2. General Considerations for Review
3. Limitations of Thermal Spray and Other Conventional Hydroxyapatite Coating Techniques
3.1. Thermal Spray
3.2. Vapor Deposition
3.3. Hot Isostatic Pressing
3.4. Sol-Gel Deposition and Dip-Coating
3.5. Electrophoretic Deposition
4. Non-Thermal Hydroxyapatite Coating Methods
4.1. Biomimetic Approach
4.1.1. Biomineralization on Phosphorylated Surface
4.1.2. Biomineralization on Carboxylated and Hydroxylated Surfaces
4.1.3. Biomineralization on Peptide-bound Surface
4.2. Direct Nanoparticle Immobilization Approach
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagho, M.M.; Hussein, E.A.; Elzatahry, A.A. Recent Overviews in Functional Polymer Composites for Biomedical Applications. Polymers 2018, 10, 739. [Google Scholar] [CrossRef] [Green Version]
- Elisseeff, J.; Guo, Q.; Lu, Q.; Madrid, M.G.; Chae, J.J. Future perspectives for regenerative medicine in ophthalmology. Middle East Afr. J. Ophthalmol. 2013, 20, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şahin, S. Use of polymers in dentistry. In Biomedical Science and Technology: Recent Developments in the Pharmaceutical and Medical Sciences; Hıncal, A.A., Kaş, H.S., Eds.; Springer US: Boston, MA, USA, 1998; pp. 163–170. [Google Scholar]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Vogler, A.E. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Cell adhesion to plasma-treated polymer surfaces. Polymer 1993, 34, 2208–2212. [Google Scholar] [CrossRef]
- Riau, A.K.; Venkatraman, S.S.; Dohlman, C.H.; Mehta, J.S. Surface Modifications of the PMMA Optic of a Keratoprosthesis to Improve Biointegration. Cornea 2017, 36, S15–S25. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.-C.; Chaâbane, C.; Gurny, R.; Bochaton-Piallat, M.-L.; Walpoth, B.; Moeller, M. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur. J. Pharm. Biopharm. 2013, 85, 78–86. [Google Scholar] [CrossRef]
- Alio, J.L.; E Mulet, M.; Haroun, H.; Merayo, J.; Moreno, J.M.R. Five year follow up of biocolonisable microporous fluorocarbon haptic (BIOKOP) keratoprosthesis implantation in patients with high risk of corneal graft failure. Br. J. Ophthalmol. 2004, 88, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 2005, 81, 1705–1728. [Google Scholar] [CrossRef]
- Lin, H.-B.; Sun, W.; Mosher, D.F.; García-Echeverría, C.; Schaufelberger, K.; Lelkes, P.I.; Cooper, S.L. Synthesis, surface, and cell-adhesion properties of polyurethanes containing covalently grafted RGD-peptides. J. Biomed. Mater. Res. 1994, 28, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Khang, G.; Lee, H.B. Interaction of Different Types of Cells on Polymer Surfaces with Wettability Gradient. J. Colloid Interface Sci. 1998, 205, 323–330. [Google Scholar] [CrossRef]
- Dunne, C.F.; Twomey, B.; Kelly, C.; Simpson, J.C.; Stanton, K.T. Hydroxyapatite and fluorapatite coatings on dental screws: Effects of blast coating process and biological response. J. Mater. Sci. Mater. Med. 2015, 26, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Jeong, K.J.; Chiang, H.H.; Zurakowski, D.; Behlau, I.; Chodosh, J.; Dohlman, C.H.; Langer, R.; Kohane, D.S. Hydroxyapatite for Keratoprosthesis Biointegration. Investig. Opthalmol. Vis. Sci. 2011, 52, 7392–7399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidigal, G.M., Jr.; Groisman, M.; de Sena, L.A.; de Almeida Soares, G. Surface Characterization of Dental Implants Coated with Hydroxyapatite by Plasma Spray and Biomimetic Process. Implant Dent. 2009, 18, 353–361. [Google Scholar] [CrossRef]
- Geesink, R.G.; De Groot, K.; Klein, C.P. Chemical implant fixation using hydroxyl-apatite coatings: The development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clin. Orthop. Relat. Res. 1987, 225, 147–170. [Google Scholar] [CrossRef]
- Vaughn, B.K.; Lombardi, A.V.; Mallory, T.H. Clinical and radiographic experience with a hydroxyapatite-coated titanium plasma-sprayed porous implant. Semin. Arthroplast. 1991, 2, 309–316. [Google Scholar]
- Mehta, J.; Futter, C.; Sandeman, S.; Faragher, R.G.A.F.; Hing, K.; Tanner, K.; Allan, B.D.S. Hydroxyapatite promotes superior keratocyte adhesion and proliferation in comparison with current keratoprosthesis skirt materials. Br. J. Ophthalmol. 2005, 89, 1356–1362. [Google Scholar] [CrossRef] [Green Version]
- Sohmura, T.; Tamasaki, H.; Ohara, T.; Takahashi, J. Calcium-phosphate surface coating by casting to improve bioactivity of titanium. J. Biomed. Mater. Res. 2001, 58, 478–485. [Google Scholar] [CrossRef]
- Riau, A.K.; Mondal, D.; Yam, G.H.F.; Setiawan, M.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S.; Venkatraman, S.S. Surface Modification of PMMA to Improve Adhesion to Corneal Substitutes in a Synthetic Core–Skirt Keratoprosthesis. ACS Appl. Mater. Interfaces 2015, 7, 21690–21702. [Google Scholar] [CrossRef]
- Kitsugi, T.; Nakamura, T.; Oka, M.; Senaha, Y.; Goto, T.; Shibuya, T. Bone-bonding behavior of plasma-sprayed coatings of BioglassR, AW-glass ceramic, and tricalcium phosphate on titanium alloy. J. Biomed. Mater. Res. 1996, 30, 261–269. [Google Scholar] [CrossRef]
- Lee, J.; Aoki, H. Hydroxyapatite Coating on Ti Plate by a Dipping Method. Bio-Medical Mater. Eng. 1995, 5, 49–58. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.; Wang, L.; Ma, X.; Huang, Y.; Qiu, Z.; Cui, F. An improved biofunction of Titanium for keratoprosthesis by hydroxyapatite-coating. J. Biomater. Appl. 2013, 28, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Riau, A.K.; Lwin, N.C.; Gelfand, L.; Hu, H.; Liedberg, B.; Chodosh, J.; Venkatraman, S.S.; Mehta, J.S. Surface modification of corneal prosthesis with nano-hydroxyapatite to enhance in vivo biointegration. Acta Biomater. 2020, 107, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating Techniques for Functional Enhancement of Metal Implants for Bone Replacement: A Review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.C.; Chang, E.; Lee, S.Y. Mechanical properties and Young’s modulus of plasma-sprayed hydroxyapatite coating on Ti substrate in simulated body fluid. J. Biomed. Mater. Res. 2003, 67, 886–899. [Google Scholar] [CrossRef]
- Cheang, P.; Khor, K. Thermal spraying of hydroxyapatite (HA) coatings: Effects of powder feedstock. J. Mater. Process. Technol. 1995, 48, 429–436. [Google Scholar] [CrossRef]
- Khor, K. Characterization of the bone-like apatite precipitated on high velocity oxy-fuel (HVOF) sprayed calcium phosphate deposits. Biomaterials 2003, 24, 769–775. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process? An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Lee, I.-S.; Kim, N.-H.; Kim, H.-E.; Jung, Y.-C.; Han, C.-H. Biological performance of calcium phosphate films formed on commercially pure Ti by electron-beam evaporation. Biomaterials 2002, 23, 609–615. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Gal-Or, L. Electrophoretic deposition of hydroxyapatite. J. Mater. Sci. Mater. Med. 1997, 8, 213–219. [Google Scholar] [CrossRef]
- Wie, H.; Herø, H.; Solheim, T. Hot isostatic pressing-processed hydroxyapatite-coated titanium implants: Light microscopic and scanning electron microscopy investigations. Int. J. Oral Maxillofac. Implant. 1998, 13, 837–844. [Google Scholar]
- Russell, S.W.; Luptak, K.A.; Suchicital, C.T.A.; Alford, T.L.; Pizziconi, V.B. Chemical and Structural Evolution of Sol-Gel-Derived Hydroxyapatite Thin Films under Rapid Thermal Processing. J. Am. Ceram. Soc. 1996, 79, 837–842. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Smith, W.F.; Hashemi, J. Polymeric materials. In Foundations of Materials Science and Engineering; Smith, W.F., Hashemi, J., Eds.; McGraw-Hill Higher Education: New York, NJ, USA, 2006; pp. 468–571. ISBN 9780073107639. [Google Scholar]
- Heimann, R.B. Plasma-Sprayed Hydroxylapatite-Based Coatings: Chemical, Mechanical, Microstructural, and Biomedical Properties. J. Therm. Spray Technol. 2016, 25, 827–850. [Google Scholar] [CrossRef] [Green Version]
- Teh, B.M.; Marano, R.J.; Shen, Y.; Friedland, P.L.; Dilley, R.J.; Atlas, M.D. Tissue Engineering of the Tympanic Membrane. Tissue Eng. Part B Rev. 2013, 19, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Nik, N.; Rad, M.R.; Nazeman, P.; Khojasteh, A. Polymers for oral and dental tissue engineering. In Biomaterials for Oral and Dental Tissue Engineering; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 25–46. [Google Scholar]
- Tevlin, R.; McArdle, A.; Atashroo, D.; Walmsley, G.; Senarath-Yapa, K.; Zielins, E.; Paik, K.; Longaker, M.; Wan, D.C. Biomaterials for Craniofacial Bone Engineering. J. Dent. Res. 2014, 93, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Brossa, F.; Cigada, A.; Chiesa, R.; Paracchini, L.; Consonni, C. Adhesion Properties of Plasma Sprayed Hydroxylapatite Coatings for Orthopaedic Prostheses. Biomed Mater. Eng. 1993, 3, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.J.; Gawne, D.T.; Dong, G. The role of grit blasting in the production of high-adhesion plasma sprayed alumina coatings. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 1997, 211, 1–9. [Google Scholar] [CrossRef]
- Costil, S.; Liao, H.; Gammoudi, A.; Coddet, C. Influence of Surface Laser Cleaning Combined with Substrate Preheating on the Splat Morphology. J. Therm. Spray Technol. 2005, 14, 31–38. [Google Scholar] [CrossRef]
- Guessasma, S.; Montavon, G.; Coddet, C. Velocity and temperature distributions of alumina–titania in-flight particles in the atmospheric plasma spray process. Surf. Coat. Technol. 2005, 192, 70–76. [Google Scholar] [CrossRef]
- Cizek, J.; Khor, K.; Procházka, Z. Influence of spraying conditions on thermal and velocity properties of plasma sprayed hydroxyapatite. Mater. Sci. Eng. C 2007, 27, 340–344. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rad, M.R.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart Coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Khor, K.; Cheang, P. Thermal sprayed hydroxyapatite splats: Nanostructures, pore formation mechanisms and TEM characterization. Biomaterials 2004, 25, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Sproul, W.D. Physical vapor deposition tool coatings. Surf. Coat. Technol. 1996, 81, 1–7. [Google Scholar] [CrossRef]
- Choi, J.-M.; Kim, H.-E.; Lee, I.-S. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials 2000, 21, 469–473. [Google Scholar] [CrossRef]
- Rajesh, P.; Muraleedharan, C.V.; Komath, M.; Varma, H. Pulsed laser deposition of hydroxyapatite on titanium substrate with titania interlayer. J. Mater. Sci. Mater. Med. 2011, 22, 497–505. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G.; Fernandes, L. On the Physical Vapour Deposition (PVD): Evolution of Magnetron Sputtering Processes for Industrial Applications. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Cabañas, M.V.; Vallet-Regi, M. Calcium phosphate coatings deposited by aerosol chemical vapour deposition. J. Mater. Chem. 2003, 13, 1104–1107. [Google Scholar] [CrossRef]
- Herø, H.; Wie, H.; Jørgensen, R.B.; Ruyter, I.E. Hydroxyapatite coatings on Ti produced by hot isostatic pressing. J. Biomed. Mater. Res. 1994, 28, 343–348. [Google Scholar] [CrossRef]
- De Groot, K.; Wolke, J.G.C.; A Jansen, J. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 137–147. [Google Scholar] [CrossRef]
- Mishra, V.K.; Rai, S.B.; Asthana, B.P.; Parkash, O.; Kumar, D. Effect of annealing on nanoparticles of hydroxyapatite synthesized via microwave irradiation: Structural and spectroscopic studies. Ceram. Int. 2014, 40, 11319–11328. [Google Scholar] [CrossRef]
- Wang, T.; Dorner-Reisel, A.; Müller, E. Thermogravimetric and thermokinetic investigation of the dehydroxylation of a hydroxyapatite powder. J. Eur. Ceram. Soc. 2004, 24, 693–698. [Google Scholar] [CrossRef]
- Nguyen, H.Q. The effect of sol–gel-formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants. Biomaterials 2004, 25, 865–876. [Google Scholar] [CrossRef]
- Liu, D.-M.; Troczynski, T.; Tseng, W.J. Water-based sol–gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Hijón, N.; Cabañas, M.V.; Peña, J.; Vallet-Regí, M. Dip coated silicon-substituted hydroxyapatite films. Acta Biomater. 2006, 2, 567–574. [Google Scholar] [CrossRef]
- Duan, K.; Tang, A.; Wang, R. Accelerating calcium phosphate growth on NaOH-treated poly-(lactic-co-glycolic acid) by evaporation-induced surface crystallization. Appl. Surf. Sci. 2008, 255, 2442–2448. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrodeposition of nanocomposite organic–inorganic coatings for biomedical applications. Int. J. Nanosci. 2005, 4, 409–418. [Google Scholar] [CrossRef]
- Baştan, F.E.; Rehman, M.A.U.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproducein vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Tas, A.C. Synthesis of biomimetic Ca-hydroxyapatite powders at 37 °C in synthetic body fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar] [CrossRef]
- Takadama, H.; Hashimoto, M.; Mizuno, M.; Kokubo, T. Round-Robin Test of SBF For In Vitro Measurement of Apatite-Forming Ability of Synthetic Materials. Phosphorus Res. Bull. 2004, 17, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Oyane, A.; Onuma, K.; Ito, A.; Kim, H.-M.; Kokubo, T.; Nakamura, T. Formation and growth of clusters in conventional and new kinds of simulated body fluids. J. Biomed. Mater. Res. 2003, 64, 339–348. [Google Scholar] [CrossRef]
- Kim, H.-M.; Miyazaki, T.; Kokubo, T.; Nakamura, T. Revised Simulated Body Fluid. Key Eng. Mater. 2000, 192, 47–50. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, X.; Chen, S.; Du, C. Osteogenic and tenogenic induction of hBMSCs by an integrated nanofibrous scaffold with chemical and structural mimicry of the bone–ligament connection. J. Mater. Chem. B 2017, 5, 1015–1027. [Google Scholar] [CrossRef]
- Tanahashi, M.; Yao, T.; Kokubo, T.; Minoda, M.; Miyamoto, T.; Nakamura, T.; Yamamura, T. Apatite coated on organic polymers by biomimetic process: Improvement in its adhesion to substrate by glow-discharge treatment. J. Biomed. Mater. Res. 1995, 29, 349–357. [Google Scholar] [CrossRef]
- Tas, A.C.; Bhaduri, S.B. Rapid coating of Ti6Al4V at room temperature with a calcium phosphate solution similar to 10× simulated body fluid. J. Mater. Res. 2004, 19, 2742–2749. [Google Scholar] [CrossRef]
- Tanahashi, M.; Matsuda, T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 1997, 34, 305–315. [Google Scholar] [CrossRef]
- Leonor, I.B.; Kim, H.-M.; Carmona, D.; Kawashita, M.; Reis, R.L.; Kokubo, T.; Nakamura, T. Functionalization of different polymers with sulfonic groups as a way to coat them with a biomimetic apatite layer. J. Mater. Sci. Mater. Electron. 2007, 18, 1923–1930. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T.; Tanihara, M.; Sakaguchi, Y.; Konagaya, S. Coating of an apatite layer on polyamide films containing sulfonic groups by a biomimetic process. Biomaterials 2004, 25, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Wentrup-Byrne, E.; Suzuki, S.; Grøndahl, L. CHAPTER 9. Biomedical Applications of Phosphorus-Containing Polymers. In Polymer Chemistry Series; Royal Society of Chemistry (RSC): London, UK, 2014; pp. 167–209. [Google Scholar]
- Tretinnikov, O.N.; Kato, K.; Ikada, Y. In vitro hydroxyapatite deposition onto a film surface-grafted with organophosphate polymer. J. Biomed. Mater. Res. 1994, 28, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Hamai, R.; Maeda, H.; Sawai, H.; Shirosaki, Y.; Kasuga, T.; Miyazaki, T. Structural effects of phosphate groups on apatite formation in a copolymer modified with Ca2+ in a simulated body fluid. J. Mater. Chem. B 2017, 6, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.; Filmon, R.; Cincu, C.; Marculescu, B.; Zaharia, C.; Tourmen, Y.; Baslé, M.; Chappard, D. Synthesis of methacryloyloxyethyl phosphate copolymers and in vitro calcification capacity. Biomaterials 2004, 25, 205–213. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Kinsella, J.M.; Murshed, M.; Cerruti, M. Surface Modification of Poly(d,l-Lactic Acid) Scaffolds for Orthopedic Applications: A Biocompatible, Nondestructive Route via Diazonium Chemistry. ACS Appl. Mater. Interfaces 2014, 6, 9975–9987. [Google Scholar] [CrossRef]
- Sailaja, G.; Sreenivasan, K.; Yokogawa, Y.; Kumary, T.; Varma, H. Bioinspired mineralization and cell adhesion on surface functionalized poly(vinyl alcohol) films. Acta Biomater. 2009, 5, 1647–1655. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Xie, C.; Zhuang, H.; Zhou, S.; Weng, J. Hydroxyapatite nucleation and growth mechanism on electrospun fibers functionalized with different chemical groups and their combinations. Biomaterials 2010, 31, 4620–4629. [Google Scholar] [CrossRef]
- Gadaleta, S.J.; Paschalis, E.P.; Betts, F.; Mendelsohn, R.; Boskey, A.L. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: New correlations between X-ray diffraction and infrared data. Calcif. Tissue Int. 1996, 58, 9–16. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Kiryukhantsev-Korneev, P.V.; Gudz, K.Y.; Konopatsky, A.S.; Polčák, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Shtansky, D.; Manakhov, A. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials 2019, 9, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, W.; Hsiong, S.; Richardson, T.; Simmons, C.A.; Mooney, D. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials 2005, 26, 303–310. [Google Scholar] [CrossRef]
- Oyane, A.; Uchida, M.; Choong, C.; Triffitt, J.; Jones, J.; Ito, A. Simple surface modification of poly(ε-caprolactone) for apatite deposition from simulated body fluid. Biomaterials 2005, 26, 2407–2413. [Google Scholar] [CrossRef]
- Qu, X.; Cui, W.; Yang, F.; Min, C.; Shen, H.; Bei, J.; Wang, S. The effect of oxygen plasma pretreatment and incubation in modified simulated body fluids on the formation of bone-like apatite on poly(lactide-co-glycolide) (70/30). Biomaterials 2007, 28, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Şeker, U.Ö.Ş.; Demir, H.V. Material Binding Peptides for Nanotechnology. Molecules 2011, 16, 1426–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Care, A.; Bergquist, P.L.; Sunna, A. Solid-binding peptides: Smart tools for nanobiotechnology. Trends Biotechnol. 2015, 33, 259–268. [Google Scholar] [CrossRef]
- Iijima, K.; Nagahama, H.; Takada, A.; Sawada, T.; Serizawa, T.; Hashizume, M. Surface functionalization of polymer substrates with hydroxyapatite using polymer-binding peptides. J. Mater. Chem. B 2016, 4, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Kumada, Y.; Murata, S.; Ishikawa, Y.; Nakatsuka, K.; Kishimoto, M. Screening of PC and PMMA-binding peptides for site-specific immobilization of proteins. J. Biotechnol. 2012, 160, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, F.; Zamanian, A.; Sahranavard, M. Mussel-inspired polydopamine-mediated surface modification of freeze-cast poly (ε-caprolactone) scaffolds for bone tissue engineering applications. Biomed. Tech. Eng. 2020, 65, 273–287. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Sun, T.; Wang, B.; Zhang, H.; Yi, W. Bioinspired Surface Functionalization for Improving Osteogenesis of Electrospun Polycaprolactone Nanofibers. Langmuir 2018, 34, 15544–15550. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Perikamana, S.K.M.; Shin, Y.M.; Lee, J.K.; Bin Lee, Y.; Heo, Y.; Ahmad, T.; Park, S.Y.; Shin, J.; Park, K.M.; Jung, H.S.; et al. Graded functionalization of biomaterial surfaces using mussel-inspired adhesive coating of polydopamine. Colloids Surf. B Biointerfaces 2017, 159, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, H.; Sjöström, M. Characterization of calcium phosphates precipitated from simulated body fluid of different buffering capacities. Biomaterials 1997, 18, 743–747. [Google Scholar] [CrossRef]
- Qu, H.; Wei, M. The effect of temperature and initial pH on biomimetic apatite coating. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 204–212. [Google Scholar] [CrossRef]
- Malakauskaite-Petruleviciene, M.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol–gel synthesis of calcium hydroxyapatite thin films on quartz substrate using dip-coating and spin-coating techniques. J. Sol-Gel Sci. Technol. 2014, 71, 437–446. [Google Scholar] [CrossRef]
- Deng, X.; Hao, J.; Wang, C. Preparation and mechanical properties of nanocomposites of poly(D,L-lactide) with Ca-deficient hydroxyapatite nanocrystals. Biomaterials 2001, 22, 2867–2873. [Google Scholar] [CrossRef]
- Riau, A.K.; Mondal, D.; Setiawan, M.; Palaniappan, A.; Yam, G.H.F.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Functionalization of the Polymeric Surface with Bioceramic Nanoparticles via a Novel, Nonthermal Dip Coating Method. ACS Appl. Mater. Interfaces 2016, 8, 35565–35577. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Barrere, F.; Van Der Valk, C.M.; Dalmeijer, R.A.J.; Van Blitterswijk, C.; De Groot, K.; Layrolle, P. In vitroandin vivodegradation of biomimetic octacalcium phosphate and carbonate apatite coatings on titanium implants. J. Biomed. Mater. Res. Part A 2002, 64, 378–387. [Google Scholar] [CrossRef]
- Riau, A.K.; Aung, T.T.; Setiawan, M.; Yang, L.; Yam, G.H.F.; Beuerman, R.W.; Venkatraman, S.S.; Mehta, J.S. Surface Immobilization of Nano-Silver on Polymeric Medical Devices to Prevent Bacterial Biofilm Formation. Pathogens 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Tan, J.; Saltzman, W.M. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat. Mater. 2004, 3, 569–574. [Google Scholar] [CrossRef]
- Liu, Y.; De Groot, K.; Hunziker, E.B. Osteoinductive implants: The mise-en-scène for drug-bearing biomimetic coatings. Ann. Biomed. Eng. 2004, 32, 398–406. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials F1185-03: Standard Specification for Composition of Hydroxylapatite for Surgical Implants. 2014. Available online: https://www.astm.org/Standards/F1185.htm (accessed on 24 October 2020).
- International Organization of Standards ISO 13779: Implants for Surgery—Hydroxyapatite. 2018. Available online: https://www.iso.org/standard/64617.html (accessed on 24 October 2020).
- McKeen, L.W. High-temperature polymers. In Effect of Temperature and Other Factors on Plastics and Elastomers; McKeen, L.W., Ed.; William Andrew Publishing: Boston, MA, USA, 2008; pp. 503–550. [Google Scholar]




| Solution | Ionic Concentration (mM) | Buffer, pH | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− | |||
| Blood plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 27.0 | 1.0 | 0.5 | - | [66] |
| Original SBF | 142.0 | 5.0 | 1.5 | 2.5 | 148.8 | 4.2 | 1.0 | 0 | * Tris, 7.25–7.4 | [65] |
| c-SBF | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 4.2 | 1.0 | 0.5 | Tris, 7.25–7.4 | [66] |
| r-SBF | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 27.0 | 1.0 | 0.5 | ** HEPES, 7.4 | [70] |
| np-SBF | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 4.2 | 1.0 | 0.5 | HEPES, 7.4 | [68] |
| t-SBF | 142.0 | 5.0 | 1.5 | 2.5 | 125.0 | 27.0 | 1.0 | 0.5 | *** dH2O | [67] |
| i-SBF | 142.0 | 5.0 | 1.0 | 1.6 | 103.0 | 27.0 | 1.0 | 0.5 | HEPES, 7.4 | [69] |
| m-SBF | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 10.0 | 1.0 | 0.5 | HEPES, 7.4 | [69] |
| 1.5× SBF | 213.0 | 7.5 | 2.3 | 3.8 | 223.0 | 6.3 | 1.5 | 0.75 | Tris, 7.25 | [72] |
| 5× SBF | 726.0 | 25.0 | 7.5 | 12.5 | 760.0 | 21.0 | 5.0 | 2.5 | Tris, 7.4 | [71] |
| 10× SBF | 1020.0 | 5.0 | 5.0 | 25.0 | 1035.0 | 10.0 | 10.0 | - | dH2O | [73] |
| Biomimetic | Direct Immobilization |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riau, A.K.; Venkatraman, S.S.; Mehta, J.S. Biomimetic vs. Direct Approach to Deposit Hydroxyapatite on the Surface of Low Melting Point Polymers for Tissue Engineering. Nanomaterials 2020, 10, 2162. https://doi.org/10.3390/nano10112162
Riau AK, Venkatraman SS, Mehta JS. Biomimetic vs. Direct Approach to Deposit Hydroxyapatite on the Surface of Low Melting Point Polymers for Tissue Engineering. Nanomaterials. 2020; 10(11):2162. https://doi.org/10.3390/nano10112162
Chicago/Turabian StyleRiau, Andri K., Subbu S. Venkatraman, and Jodhbir S. Mehta. 2020. "Biomimetic vs. Direct Approach to Deposit Hydroxyapatite on the Surface of Low Melting Point Polymers for Tissue Engineering" Nanomaterials 10, no. 11: 2162. https://doi.org/10.3390/nano10112162
APA StyleRiau, A. K., Venkatraman, S. S., & Mehta, J. S. (2020). Biomimetic vs. Direct Approach to Deposit Hydroxyapatite on the Surface of Low Melting Point Polymers for Tissue Engineering. Nanomaterials, 10(11), 2162. https://doi.org/10.3390/nano10112162