Effect of Surfactant Polyvinyl Pyrrolidone on the Properties of Microporous Carbon Nanospheres Reinforced Magnesium Matrix Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Processing
2.2.1. Preparation of Carbon-Reinforced Mg Composite Powders without PVP Pretreatment
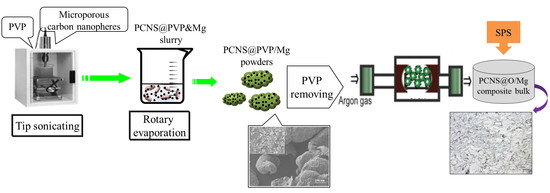
2.2.2. Preparation of Carbon-Reinforced Mg Composite Powders with PVP Pretreatment
2.2.3. PVP Removal Treatment
2.2.4. Processing of Carbon-Reinforced Mg Composite Bulks
2.3. Characterization
3. Results and Discussion
3.1. Influence of PVP on Reinforcement Dispersion
3.2. Influence of PVP on Composite Powders
3.3. Influence of PVP on Optical Micrographs of Bulk Composites
3.4. Interfacial Microstructure
3.5. Tensile Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lan, J.; Yang, Y.; Li, X.-C. Microstructure and microhardness of SiC nanoparticles reinforced magnesium composites fabricated by ultrasonic method. Mater. Sci. Eng. A 2004, 386, 284–291. [Google Scholar] [CrossRef]
- Cao, G.; Kobliska, J.; Konishi, H.; Li, X. Tensile properties and microstructure of SiC nanoparticle–reinforced Mg-4Zn alloy fabricated by ultrasonic cavitation–based solidification processing. Metall. Mater. Trans. A 2008, 39, 880–886. [Google Scholar] [CrossRef]
- Cao, G.; Konishi, H.; Li, X. Mechanical properties and microstructure of SiC-reinforced Mg-(2,4)Al-1Si nanocomposites fabricated by ultrasonic cavitation based solidification processing. Mater. Sci. Eng. A 2008, 486, 357–362. [Google Scholar] [CrossRef]
- Wang, Y.; Korai, Y.; Mochida, I. Carbon disc of high density and strength prepared from synthetic pitch-derived mesocarbon microbeads. Carbon 1999, 7, 1049–1057. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, C.; Kang, J.; Cho, Y.; Moon, J.-H. Synthesis of porous carbon balls from spherical colloidal crystal templates. Langmuir 2012, 28, 10543–10550. [Google Scholar] [CrossRef]
- Titirici, M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef]
- Peng, T.; Chang, I. Uniformly dispersion of carbon nanotube in aluminum powders by wet shake-mixing approach. Powder Technol 2015, 284, 32–39. [Google Scholar] [CrossRef]
- Gao, X.; Yue, H.-Y.; Guo, E.-J.; Zhang, H.; Lin, X.-Y.; Yao, L.H.; Wang, B. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites. Mater. Design 2016, 94, 54–60. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Z.-Q.; Fan, G.-L.; Cao, L.-L.; Zhang, D. Strong and ductile carbon nanotube/aluminum bulk nanolaminated composites with two-dimensional alignment of carbon nanotubes. Scr. Mater. 2012, 6, 331–334. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, G.-L.; Li, Z.-Q.; Kai, Z.-X.; Zhang, D.; Chen, Z.-X.; Humphries, S.; Heness, G.; Yeung, W.-Y. An approach to the uniform dispersion of a high volume fraction of carbon nanotubes in aluminum powder. Carbon 2011, 49, 1965–1971. [Google Scholar] [CrossRef]
- Jiang, R.-R.; Zhou, X.-F.; Fang, Q.-L.; Liu, Z.-P. Copper-graphene bulk composites with homogeneous graphene dispersion and enhanced mechanical properties. Mater. Sci. Eng. A 2016, 654, 124–130. [Google Scholar] [CrossRef]
- Jiang, C.-J.; Duan, Z.-W.; Zhang, Z.-Z.; Wang, C. Effect of surfactants on dispersing properties in alcohol solvent for silver nanopowders. Rare Met. Mater. Eng. 2007, 4, 724–727. [Google Scholar]
- Nan, Q. Study on the Dispersion of Noncovalent Functionalization of Carbon Nanotubes in Solvents and Polymers. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2017. [Google Scholar]
- Wajid, A.-S.; Das, S.; Irin, F.; Ahmed, H.-S.; Shelburne, J.-L.; Parviz, D.; Fullerton, R.-J.; Jankowski, A.-F.; Hedden, R.-C.; Green, M.-J. Polymer-stabilized graphene dispersions at high concentrations in organic solvents for composite production. Carbon 2012, 50, 526–534. [Google Scholar] [CrossRef]
- Jin, L.; Yang, Y.-Z.; Fan, J.-F.; Xu, B.-S. Improved dispersion of carbon microspheres reinforcement in Mg matrix composites. J. Taiyuan. Univ. Technol. 2020, 51, 363–371. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Yang, Y.-Z.; Liu, X.-G.; Xu, B.-S. Preparation of surface molecularly imprinted matrix materials porous carbon microspheres from glucose by hydrothermal carbonization method. China Sci. 2012, 7, 898–903. [Google Scholar]
- Pandiyarajan, T.; Karthikeyan, B. Structural, thermal and optical properties of PVP capped ZnO films. Adv. Mater. Res. 2013, 678, 253–257. [Google Scholar] [CrossRef]
- Jin, Y.-Z.; Gao, C.; Hsu, W.-K.; Zhu, Y.-Q.; Huczko, A.; Bystrzejewski, M.; Roe, M.; Lee, C.; Acquah, S.; Kroto, H.; et al. Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 2005, 9, 1944–1953. [Google Scholar] [CrossRef]
- Ma, A.-L.; Wang, X.-M.; Li, T.-B.; Liu, X.-G.; Xu, B.-S. Characteristics of carbon microspheres and study on its adsorption isotherms. Mater. Sci. Eng. 2007, 443, 54–59. [Google Scholar] [CrossRef]
- Wang, X.-J.; Chen, D.-J.; Zhou, J.-J. Synthesis and characterization of the carbon nanotubes/magnesium oxide nanocomposite by precursor decomposing method. New Chem. Mater. 2009, 2, 35–37. [Google Scholar]
- Chang, S.; Lee, S.; Kang, K.-M.; Kamado, S.; Kojima, Y. Improvement of mechanical characteristics in severely plastic-deformed Mg alloys. Mater. Trans. 2004, 45, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Ryou, J.; Hong, S. First-principles study of carbon atoms adsorbed on MgO(100) related to graphene growth. Curr. Appl. Phys. 2013, 13, 327–330. [Google Scholar] [CrossRef]
- Yuan, Q.-H.; Zeng, X.-S.; Liu, Y.; Luo, L.; Wu, J.-B.; Wang, Y.-C.; Zhou, G.-H. Microstructure and mechanical properties of AZ91 alloy reinforced by carbon nanotubes coated with MgO. Carbon 2016, 96, 843–855. [Google Scholar] [CrossRef]
- Jin, L.; Yang, Y.-Z.; Fan, J.-F.; Xu, B.-S. Effect of surface functionalization on properties of carbon microspheres reinforced magnesium composites. Mater. Rev. 2020, 8. accepted. [Google Scholar]
- Jerng, S.; Lee, J.; Yu, D.; Kim, Y.; Ryou, J.; Hong, S.; Kim, C.; Yoon, S.; Chun, S. Graphitic carbon growth on MgO(100) by molecular beam epitaxy. J. Phys. Chem. C 2012, 116, 7380–7385. [Google Scholar] [CrossRef]
- Kondoh, K.; Fukuda, H.; Umeda, J.; Imai, H.; Fugetsu, B.; Endo, M. Microstructural and mechanical analysis of carbon nanotube reinforced magnesium alloy powder composites. Mater. Sci. Eng. A 2010, 527, 4103–4108. [Google Scholar] [CrossRef]
- Yan, Y. Mg Matrix Composites Reinforced with Nano Particles Fabricated by SPS Followed by Hot Extrusion. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2017. [Google Scholar]










| Mass Percentage of PCNS (wt.%) | Bulk Sample |
|---|---|
| 0.5 | 0.5 PCNS/Mg |
| 1 | 1 PCNS/Mg |
| Mass Percentage of PCNS (wt.%) | Bulk Sample | Bulk Sample of Removing PVP |
|---|---|---|
| 0.5 | 0.5 PCNS@PVP/Mg | 0.5 PCNS@O/Mg |
| 1 | 1 PCNS@PVP/Mg | 1 PCNS@O/Mg |
| 2 | 2 PCNS@PVP/Mg | 2 PCNS@O/Mg |
| 4 | 4 PCNS@PVP/Mg | 4 PCNS@O/Mg |
| Sample | Mass Percentage of PCNS (wt.%) | σ0.2 (MPa) | σUTS (MPa) | ε (%) |
|---|---|---|---|---|
| Mg | 0 | 100 | 149 | 6.4 |
| 0.5 PCNS/Mg | 0.5 | 140 | 227 | 4.8 |
| 1 PCNS/Mg | 1 | 142 | 201 | 2.6 |
| 0.5 PCNS@PVP/Mg | 0.5 | 150 | 186 | 3.2 |
| 1 PCNS@PVP/Mg | 1 | 145 | 170 | 2.4 |
| 2 PCNS@PVP/Mg | 2 | 165 | 209 | 2.7 |
| 4 PCNS@PVP/Mg | 4 | 93 | 98 | 1.1 |
| 0.5 PCNS@O/Mg | 0.5 | 148 | 193 | 3.7 |
| 1 PCNS@O/Mg | 1 | 166 | 204 | 3.1 |
| 2 PCNS@O/Mg | 2 | 177 | 206 | 3.4 |
| 4 PCNS@O/Mg | 4 | 147 | 169 | 2.2 |
| Sample | Tensile Yield Strength (MPa) | True Density (g/cm3) | Strength to-Weight Ratio (N∙m/kg) |
|---|---|---|---|
| Mg | 100 | 1.7362 | 57.6 × 103 |
| 0.5 PCNS@O/Mg | 148 | 1.7381 | 85.2 × 103 |
| 1 PCNS@O/Mg | 166 | 1.7319 | 95.8 × 103 |
| 2 PCNS@O/Mg | 177 | 1.7282 | 102.4 × 103 |
| 4 PCNS@O/Mg | 147 | 1.7101 | 86.0 × 103 |
| Mg-1Al-0.3CNTs [27] | 166 | 1.7443 | 95.2 × 103 |
| Mg-1Al-0.6CNTs [27] | 161 | 1.7400 | 92.5 × 103 |
| Mg-1Al-0.6SiC [27] | 156 | 1.7557 | 88.8 × 103 |
| Mg-1Al-1.2SiC [27] | 176 | 1.7646 | 99.7 × 103 |
| Mg-1Al-2.4SiC [27] | 186 | 1.7679 | 95.0 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Yang, Y.-Z.; Fan, J.-F.; Xu, B.-S. Effect of Surfactant Polyvinyl Pyrrolidone on the Properties of Microporous Carbon Nanospheres Reinforced Magnesium Matrix Composites. Nanomaterials 2020, 10, 2281. https://doi.org/10.3390/nano10112281
Jin L, Yang Y-Z, Fan J-F, Xu B-S. Effect of Surfactant Polyvinyl Pyrrolidone on the Properties of Microporous Carbon Nanospheres Reinforced Magnesium Matrix Composites. Nanomaterials. 2020; 10(11):2281. https://doi.org/10.3390/nano10112281
Chicago/Turabian StyleJin, Lin, Yong-Zhen Yang, Jian-Feng Fan, and Bing-She Xu. 2020. "Effect of Surfactant Polyvinyl Pyrrolidone on the Properties of Microporous Carbon Nanospheres Reinforced Magnesium Matrix Composites" Nanomaterials 10, no. 11: 2281. https://doi.org/10.3390/nano10112281
APA StyleJin, L., Yang, Y. -Z., Fan, J. -F., & Xu, B. -S. (2020). Effect of Surfactant Polyvinyl Pyrrolidone on the Properties of Microporous Carbon Nanospheres Reinforced Magnesium Matrix Composites. Nanomaterials, 10(11), 2281. https://doi.org/10.3390/nano10112281